Helicobacter species are potent drivers of colonic T cell responses in homeostasis and inflammation
- PMID: 28733471
- PMCID: PMC5684094
- DOI: 10.1126/sciimmunol.aal5068
Helicobacter species are potent drivers of colonic T cell responses in homeostasis and inflammation
Abstract
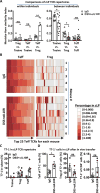
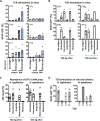
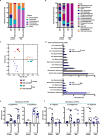
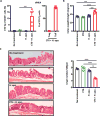
Specific gut commensal bacteria improve host health by eliciting mutualistic regulatory T (Treg) cell responses. However, the bacteria that induce effector T (Teff) cells during inflammation are unclear. We addressed this by analyzing bacterial-reactive T cell receptor (TCR) transgenic cells and TCR repertoires in a murine colitis model. Unexpectedly, we found that mucosal-associated Helicobacter species triggered both Treg cell responses during homeostasis and Teff cell responses during colitis, as suggested by an increased overlap between the Teff/Treg TCR repertoires with colitis. Four of six Treg TCRs tested recognized mucosal-associated Helicobacter species in vitro and in vivo. By contrast, the marked expansion of luminal Bacteroides species seen during colitis did not trigger a commensurate Teff cell response. Unlike other Treg cell-inducing bacteria, Helicobacter species are known pathobionts and cause disease in immunodeficient mice. Thus, our study suggests a model in which mucosal bacteria elicit context-dependent Treg or Teff cell responses to facilitate intestinal tolerance or inflammation.
Copyright © 2017 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



Similar articles
-
Clonotype-specific avidity influences the dynamics and hierarchy of virus-specific regulatory and effector CD4(+) T-cell responses.Eur J Immunol. 2014 Apr;44(4):1058-68. doi: 10.1002/eji.201343766. Epub 2014 Feb 8. Eur J Immunol. 2014. PMID: 24510524
-
Differential effects of α4β7 and GPR15 on homing of effector and regulatory T cells from patients with UC to the inflamed gut in vivo.Gut. 2016 Oct;65(10):1642-64. doi: 10.1136/gutjnl-2015-310022. Epub 2015 Jul 24. Gut. 2016. PMID: 26209553 Free PMC article.
-
Thymic commitment of regulatory T cells is a pathway of TCR-dependent selection that isolates repertoires undergoing positive or negative selection.Curr Top Microbiol Immunol. 2005;293:43-71. doi: 10.1007/3-540-27702-1_3. Curr Top Microbiol Immunol. 2005. PMID: 15981475 Review.
-
Expression of dual TCR on DO11.10 T cells allows for ovalbumin-induced oral tolerance to prevent T cell-mediated colitis directed against unrelated enteric bacterial antigens.J Immunol. 2004 Feb 1;172(3):1515-23. doi: 10.4049/jimmunol.172.3.1515. J Immunol. 2004. PMID: 14734729
-
Development, validation and implementation of an in vitro model for the study of metabolic and immune function in normal and inflamed human colonic epithelium.Dan Med J. 2015 Jan;62(1):B4973. Dan Med J. 2015. PMID: 25557335 Review.
Cited by
-
Intestinal microbes direct CX3CR1+ cells to balance intestinal immunity.Gut Microbes. 2019 Jul 4;10(4):540-546. doi: 10.1080/19490976.2018.1559683. Epub 2019 Jan 6. Gut Microbes. 2019. PMID: 33970784 Free PMC article.
-
A RORγt+ cell instructs gut microbiota-specific Treg cell differentiation.Nature. 2022 Oct;610(7933):737-743. doi: 10.1038/s41586-022-05089-y. Epub 2022 Sep 7. Nature. 2022. PMID: 36071167 Free PMC article.
-
Regulatory T Cells Developing Peri-Weaning Are Continually Required to Restrain Th2 Systemic Responses Later in Life.Front Immunol. 2021 Feb 3;11:603059. doi: 10.3389/fimmu.2020.603059. eCollection 2020. Front Immunol. 2021. PMID: 33613522 Free PMC article.
-
Endocytosis of commensal antigens by intestinal epithelial cells regulates mucosal T cell homeostasis.Science. 2019 Mar 8;363(6431):eaat4042. doi: 10.1126/science.aat4042. Science. 2019. PMID: 30846568 Free PMC article.
-
Environmental enteric dysfunction induces regulatory T cells that inhibit local CD4+ T cell responses and impair oral vaccine efficacy.Immunity. 2021 Aug 10;54(8):1745-1757.e7. doi: 10.1016/j.immuni.2021.07.005. Epub 2021 Aug 3. Immunity. 2021. PMID: 34348118 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

