Regulation of inflammation by microbiota interactions with the host
- PMID: 28722709
- PMCID: PMC5800875
- DOI: 10.1038/ni.3780
Regulation of inflammation by microbiota interactions with the host
Abstract
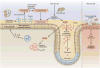
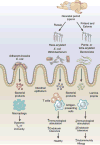
The study of the intestinal microbiota has begun to shift from cataloging individual members of the commensal community to understanding their contributions to the physiology of the host organism in health and disease. Here, we review the effects of the microbiome on innate and adaptive immunological players from epithelial cells and antigen-presenting cells to innate lymphoid cells and regulatory T cells. We discuss recent studies that have identified diverse microbiota-derived bioactive molecules and their effects on inflammation within the intestine and distally at sites as anatomically remote as the brain. Finally, we highlight new insights into how the microbiome influences the host response to infection, vaccination and cancer, as well as susceptibility to autoimmune and neurodegenerative disorders.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


Similar articles
-
Immune-microbiota interactions in health and disease.Clin Immunol. 2015 Aug;159(2):122-127. doi: 10.1016/j.clim.2015.05.014. Epub 2015 Jun 30. Clin Immunol. 2015. PMID: 26141651 Free PMC article. Review.
-
Innate and adaptive lymphocytes sequentially shape the gut microbiota and lipid metabolism.Nature. 2018 Feb 8;554(7691):255-259. doi: 10.1038/nature25437. Epub 2018 Jan 22. Nature. 2018. PMID: 29364878
-
Commensal bacteria (normal microflora), mucosal immunity and chronic inflammatory and autoimmune diseases.Immunol Lett. 2004 May 15;93(2-3):97-108. doi: 10.1016/j.imlet.2004.02.005. Immunol Lett. 2004. PMID: 15158604 Review.
-
Microbiota-myeloid cell crosstalk beyond the gut.J Leukoc Biol. 2016 Nov;100(5):865-879. doi: 10.1189/jlb.3RI0516-222R. Epub 2016 Sep 7. J Leukoc Biol. 2016. PMID: 27605211 Free PMC article. Review.
-
The Probiotic Compound VSL#3 Modulates Mucosal, Peripheral, and Systemic Immunity Following Murine Broad-Spectrum Antibiotic Treatment.Front Cell Infect Microbiol. 2017 May 5;7:167. doi: 10.3389/fcimb.2017.00167. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 28529928 Free PMC article.
Cited by
-
Interaction between intestinal mycobiota and microbiota shapes lung inflammation.Imeta. 2024 Sep 14;3(5):e241. doi: 10.1002/imt2.241. eCollection 2024 Oct. Imeta. 2024. PMID: 39429884 Free PMC article.
-
Modulation of liver metabolism and gut microbiota by Alhagi-honey alleviated heat stress-induced liver damage.Stress Biol. 2024 Sep 30;4(1):41. doi: 10.1007/s44154-024-00178-6. Stress Biol. 2024. PMID: 39347852 Free PMC article.
-
Causal associations between gut microbiota, circulating inflammatory proteins, and epilepsy: a multivariable Mendelian randomization study.Front Immunol. 2024 Sep 9;15:1438645. doi: 10.3389/fimmu.2024.1438645. eCollection 2024. Front Immunol. 2024. PMID: 39315097 Free PMC article.
-
The involvement of effector memory CD4+ T cells in mediating the impact of genus Oscillibacter gut microbiota on Alzheimer's disease: a Mendelian randomization study.Front Aging Neurosci. 2024 Aug 7;16:1423707. doi: 10.3389/fnagi.2024.1423707. eCollection 2024. Front Aging Neurosci. 2024. PMID: 39170894 Free PMC article.
-
Association between gut microbiota and diabetic microvascular complications: a two-sample Mendelian randomization study.Front Endocrinol (Lausanne). 2024 Aug 2;15:1364280. doi: 10.3389/fendo.2024.1364280. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 39157683 Free PMC article.
References
-
- Sender R, Fuchs S, Milo R. Are we really vastly outnumbered? Revisiting the ratio of bacterial to host cells in humans. Cell. 2016;164:337–340. - PubMed
-
- Gilbert JA, et al. Microbiome-wide association studies link dynamic microbial consortia to disease. Nature. 2016;535:94–103. - PubMed
-
- Honda K, Littman DR. The microbiota in adaptive immune homeostasis and disease. Nature. 2016;535:75–84. - PubMed
Publication types
MeSH terms
Grants and funding
- R01 AI074878/AI/NIAID NIH HHS/United States
- R21 DK110262/DK/NIDDK NIH HHS/United States
- R21 CA201829/CA/NCI NIH HHS/United States
- R00 DK098310/DK/NIDDK NIH HHS/United States
- R01 AI095466/AI/NIAID NIH HHS/United States
- R01 DK111862/DK/NIDDK NIH HHS/United States
- R21 AI123819/AI/NIAID NIH HHS/United States
- R01 AI123368/AI/NIAID NIH HHS/United States
- DP5 OD012116/OD/NIH HHS/United States
- R01 AI127658/AI/NIAID NIH HHS/United States
- P01 DK072201/DK/NIDDK NIH HHS/United States
- R01 AI102942/AI/NIAID NIH HHS/United States
- R01 AI073899/AI/NIAID NIH HHS/United States
- K08 DK099381/DK/NIDDK NIH HHS/United States
- R21 AI087990/AI/NIAID NIH HHS/United States
- K99 DK098310/DK/NIDDK NIH HHS/United States
- R01 AI095245/AI/NIAID NIH HHS/United States
- R21 AI083480/AI/NIAID NIH HHS/United States
- U01 AI095608/AI/NIAID NIH HHS/United States
- R01 AI061570/AI/NIAID NIH HHS/United States
- R01 AI097333/AI/NIAID NIH HHS/United States
- R56 AI073899/AI/NIAID NIH HHS/United States
- R01 AI123284/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

