SIRT3 Enhances Mesenchymal Stem Cell Longevity and Differentiation
- PMID: 28717408
- PMCID: PMC5499245
- DOI: 10.1155/2017/5841716
SIRT3 Enhances Mesenchymal Stem Cell Longevity and Differentiation
Abstract
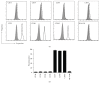
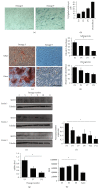
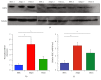
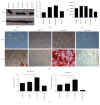
Mesenchymal stem cells (MSCs) are multipotent cells that are currently being investigated in a wide variety of clinical trials for their anti-inflammatory and immunomodulatory properties as well as their osteogenic and chondrogenic capabilities. However, there are considerable interdonor variability and heterogeneity of MSC populations, making it challenging to compare different products. Furthermore, proliferation, differentiation, and immunomodulation of MSCs decrease with aging and ex vivo expansion. The sirtuins have emerged as a class of protein deacylases involved in aging, oxidative stress, and metabolism. Sirtuin 3 (SIRT3) is the major mitochondrial deacetylase involved in reducing oxidative stress while preserving oxidative metabolism, and its levels have been shown to decrease with age. This study investigated the role of SIRT3 in MSC differentiation and aging. As MSCs were expanded ex vivo, SIRT3 levels decreased. In addition, SIRT3 depletion reduced MSC differentiation into adipocytes and osteoblasts. Furthermore, overexpression of SIRT3 in later-passage MSCs reduced aging-related senescence, reduced oxidative stress, and enhanced their ability to differentiate. These data suggest that overexpressing SIRT3 might represent a strategy to increase the quality and quantity of MSCs utilized for clinical applications.
Figures
Similar articles
-
Aging impairs beige adipocyte differentiation of mesenchymal stem cells via the reduced expression of Sirtuin 1.Biochem Biophys Res Commun. 2018 Jun 7;500(3):682-690. doi: 10.1016/j.bbrc.2018.04.136. Biochem Biophys Res Commun. 2018. PMID: 29678576
-
Effects of Oxidative Stress on Mesenchymal Stem Cell Biology.Oxid Med Cell Longev. 2016;2016:2989076. doi: 10.1155/2016/2989076. Epub 2016 Jun 16. Oxid Med Cell Longev. 2016. PMID: 27413419 Free PMC article. Review.
-
EphB2 signaling-mediated Sirt3 expression reduces MSC senescence by maintaining mitochondrial ROS homeostasis.Free Radic Biol Med. 2017 Sep;110:368-380. doi: 10.1016/j.freeradbiomed.2017.07.001. Epub 2017 Jul 4. Free Radic Biol Med. 2017. PMID: 28687409
-
Nicotinamide Mononucleotide Supplementation Improves Mitochondrial Dysfunction and Rescues Cellular Senescence by NAD+/Sirt3 Pathway in Mesenchymal Stem Cells.Int J Mol Sci. 2022 Nov 25;23(23):14739. doi: 10.3390/ijms232314739. Int J Mol Sci. 2022. PMID: 36499074 Free PMC article.
-
Optimization of oxidative stress for mesenchymal stromal/stem cell engraftment, function and longevity.Free Radic Biol Med. 2021 May 1;167:193-200. doi: 10.1016/j.freeradbiomed.2021.02.042. Epub 2021 Mar 4. Free Radic Biol Med. 2021. PMID: 33677063 Review.
Cited by
-
Melatonin restores the osteoporosis-impaired osteogenic potential of bone marrow mesenchymal stem cells by preserving SIRT1-mediated intracellular antioxidant properties.Free Radic Biol Med. 2020 Jan;146:92-106. doi: 10.1016/j.freeradbiomed.2019.10.412. Epub 2019 Oct 24. Free Radic Biol Med. 2020. PMID: 31669348 Free PMC article.
-
Mitochondrial Sirtuin 3: New emerging biological function and therapeutic target.Theranostics. 2020 Jul 9;10(18):8315-8342. doi: 10.7150/thno.45922. eCollection 2020. Theranostics. 2020. PMID: 32724473 Free PMC article. Review.
-
Sirt3 Promotes Chondrogenesis, Chondrocyte Mitochondrial Respiration and the Development of High-Fat Diet-Induced Osteoarthritis in Mice.J Bone Miner Res. 2022 Dec;37(12):2531-2547. doi: 10.1002/jbmr.4721. Epub 2022 Oct 27. J Bone Miner Res. 2022. PMID: 36214465 Free PMC article.
-
Deacetylation of FOXP1 by HDAC7 potentiates self-renewal of mesenchymal stem cells.Stem Cell Res Ther. 2023 Jul 28;14(1):188. doi: 10.1186/s13287-023-03376-7. Stem Cell Res Ther. 2023. PMID: 37507770 Free PMC article.
-
Senescence in Mesenchymal Stem Cells: Functional Alterations, Molecular Mechanisms, and Rejuvenation Strategies.Front Cell Dev Biol. 2020 May 5;8:258. doi: 10.3389/fcell.2020.00258. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32478063 Free PMC article. Review.
References
-
- Stagg J., Galipeau J. Mechanisms of immune modulation by mesenchymal stromal cells and clinical translation. Current Molecular Medicine. 2013;13(5):856–867. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

