Insights into the Development of the Adult Leydig Cell Lineage from Stem Leydig Cells
- PMID: 28701961
- PMCID: PMC5487449
- DOI: 10.3389/fphys.2017.00430
Insights into the Development of the Adult Leydig Cell Lineage from Stem Leydig Cells
Abstract
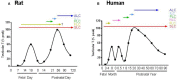
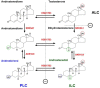
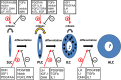
Adult Leydig cells (ALCs) are the steroidogenic cells in the testes that produce testosterone. ALCs develop postnatally from a pool of stem cells, referred to as stem Leydig cells (SLCs). SLCs are spindle-shaped cells that lack steroidogenic cell markers, including luteinizing hormone (LH) receptor and 3β-hydroxysteroid dehydrogenase. The commitment of SLCs into the progenitor Leydig cells (PLCs), the first stage in the lineage, requires growth factors, including Dessert Hedgehog (DHH) and platelet-derived growth factor-AA. PLCs are still spindle-shaped, but become steroidogenic and produce mainly androsterone. The next transition in the lineage is from PLC to the immature Leydig cell (ILC). This transition requires LH, DHH, and androgen. ILCs are ovoid cells that are competent for producing a different form of androgen, androstanediol. The final stage in the developmental lineage is ALC. The transition to ALC involves the reduced expression of 5α-reductase 1, a step that is necessary to make the cells to produce testosterone as the final product. The transitions along the Leydig cell lineage are associated with the progressive down-regulation of the proliferative activity, and the up-regulation of steroidogenic capacity, with each step requiring unique regulatory signaling.
Keywords: Desert Hedgehog; Leydig cells; development; steroidogenic factor 1; testosterone.
Figures
Similar articles
-
Gene expression during development of fetal and adult Leydig cells.Ann N Y Acad Sci. 2007 Dec;1120:16-35. doi: 10.1196/annals.1411.016. Ann N Y Acad Sci. 2007. PMID: 18184909
-
Deletion of the Igf1 gene: suppressive effects on adult Leydig cell development.J Androl. 2010 Jul-Aug;31(4):379-87. doi: 10.2164/jandrol.109.008680. Epub 2010 Mar 4. J Androl. 2010. PMID: 20203337 Free PMC article.
-
Decreased cyclin A2 and increased cyclin G1 levels coincide with loss of proliferative capacity in rat Leydig cells during pubertal development.Endocrinology. 1997 Sep;138(9):3719-26. doi: 10.1210/endo.138.9.5387. Endocrinology. 1997. PMID: 9275057
-
Stem Leydig Cells in the Adult Testis: Characterization, Regulation and Potential Applications.Endocr Rev. 2020 Feb 1;41(1):22-32. doi: 10.1210/endrev/bnz013. Endocr Rev. 2020. PMID: 31673697 Free PMC article. Review.
-
Leydig cell stem cells: Identification, proliferation and differentiation.Mol Cell Endocrinol. 2017 Apr 15;445:65-73. doi: 10.1016/j.mce.2016.10.010. Epub 2016 Oct 12. Mol Cell Endocrinol. 2017. PMID: 27743991 Free PMC article. Review.
Cited by
-
Mechanisms of Cadmium-Induced Testicular Injury: A Risk to Male Fertility.Cells. 2022 Nov 14;11(22):3601. doi: 10.3390/cells11223601. Cells. 2022. PMID: 36429028 Free PMC article. Review.
-
Comparative testis structure and function in three representative mice strains.Cell Tissue Res. 2020 Nov;382(2):391-404. doi: 10.1007/s00441-020-03239-0. Epub 2020 Jul 14. Cell Tissue Res. 2020. PMID: 32666138
-
TCF21+ mesenchymal cells contribute to testis somatic cell development, homeostasis, and regeneration in mice.Nat Commun. 2021 Jun 23;12(1):3876. doi: 10.1038/s41467-021-24130-8. Nat Commun. 2021. PMID: 34162856 Free PMC article.
-
Loss of PBX1 function in Leydig cells causes testicular dysgenesis and male sterility.Cell Mol Life Sci. 2024 May 9;81(1):212. doi: 10.1007/s00018-024-05249-5. Cell Mol Life Sci. 2024. PMID: 38724675 Free PMC article.
-
STARD1 Functions in Mitochondrial Cholesterol Metabolism and Nascent HDL Formation. Gene Expression and Molecular mRNA Imaging Show Novel Splicing and a 1:1 Mitochondrial Association.Front Endocrinol (Lausanne). 2020 Oct 20;11:559674. doi: 10.3389/fendo.2020.559674. eCollection 2020. Front Endocrinol (Lausanne). 2020. PMID: 33193082 Free PMC article. Review.
References
-
- Agarwal A. K., Monder C., Eckstein B., White P. C. (1989). Cloning and expression of rat cDNA encoding corticosteroid 11β-dehydrogenase. J. Biol. Chem. 264, 18939–18943. - PubMed
-
- Augustowska K., Gregoraszczuk E. E., Grochowalski A., Milewicz T., Mika M., Krzysiek J., et al. . (2003a). Comparison of accumulation and altered steroid secretion by placental tissue treated with TCDD and natural mixture of PCDDs-PCDFs. Reproduction 126, 681–687. 10.1530/rep.0.1260681 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

