TRIM21 is critical for survival of Toxoplasma gondii infection and localises to GBP-positive parasite vacuoles
- PMID: 28701773
- PMCID: PMC5507857
- DOI: 10.1038/s41598-017-05487-7
TRIM21 is critical for survival of Toxoplasma gondii infection and localises to GBP-positive parasite vacuoles
Abstract
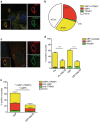
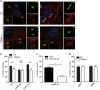
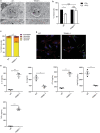
Interferon gamma (IFNγ) is the major proinflammatory cytokine conferring resistance to the intracellular vacuolar pathogen Toxoplasma gondii by inducing the destruction of the parasitophorous vacuole (PV). We previously identified TRIM21 as an IFNγ-driven E3 ubiquitin ligase mediating the deposition of ubiquitin around pathogen inclusions. Here, we show that TRIM21 knockout mice were highly susceptible to Toxoplasma infection, exhibiting decreased levels of serum inflammatory cytokines and higher parasite burden in the peritoneum and brain. We demonstrate that IFNγ drives recruitment of TRIM21 to GBP1-positive Toxoplasma vacuoles, leading to Lys63-linked ubiquitination of the vacuole and restriction of parasite early replication without interfering with vacuolar disruption. As seen in vivo, TRIM21 impacted the secretion of inflammatory cytokines. This study identifies TRIM21 as a previously unknown modulator of Toxoplasma gondii resistance in vivo thereby extending host innate immune recognition of eukaryotic pathogens to include E3 ubiquitin ligases.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
K63-Linked Ubiquitination Targets Toxoplasma gondii for Endo-lysosomal Destruction in IFNγ-Stimulated Human Cells.PLoS Pathog. 2016 Nov 22;12(11):e1006027. doi: 10.1371/journal.ppat.1006027. eCollection 2016 Nov. PLoS Pathog. 2016. PMID: 27875583 Free PMC article.
-
Murine Gbp1 and Gbp2 are ubiquitinated independent of Toxoplasma gondii infection.BMC Res Notes. 2018 Mar 6;11(1):166. doi: 10.1186/s13104-018-3267-z. BMC Res Notes. 2018. PMID: 29510761 Free PMC article.
-
Essential Role of mGBP7 for Survival of Toxoplasma gondii Infection.mBio. 2020 Jan 21;11(1):e02993-19. doi: 10.1128/mBio.02993-19. mBio. 2020. PMID: 31964735 Free PMC article.
-
Exposing Toxoplasma gondii hiding inside the vacuole: a role for GBPs, autophagy and host cell death.Curr Opin Microbiol. 2017 Dec;40:72-80. doi: 10.1016/j.mib.2017.10.021. Epub 2017 Nov 12. Curr Opin Microbiol. 2017. PMID: 29141239 Free PMC article. Review.
-
Biogenesis of and activities at the Toxoplasma gondii parasitophorous vacuole membrane.Subcell Biochem. 2008;47:155-64. doi: 10.1007/978-0-387-78267-6_12. Subcell Biochem. 2008. PMID: 18512349 Review.
Cited by
-
Ehrlichia chaffeensis Outer Membrane Protein 1-Specific Human Antibody-Mediated Immunity Is Defined by Intracellular TRIM21-Dependent Innate Immune Activation and Extracellular Neutralization.Infect Immun. 2019 Nov 18;87(12):e00383-19. doi: 10.1128/IAI.00383-19. Print 2019 Dec. Infect Immun. 2019. PMID: 31548319 Free PMC article.
-
Interferon-λ3 Promotes Epithelial Defense and Barrier Function Against Cryptosporidium parvum Infection.Cell Mol Gastroenterol Hepatol. 2019;8(1):1-20. doi: 10.1016/j.jcmgh.2019.02.007. Epub 2019 Mar 5. Cell Mol Gastroenterol Hepatol. 2019. PMID: 30849550 Free PMC article.
-
Lessons from Toxoplasma: Host responses that mediate parasite control and the microbial effectors that subvert them.J Exp Med. 2021 Nov 1;218(11):e20201314. doi: 10.1084/jem.20201314. Epub 2021 Oct 20. J Exp Med. 2021. PMID: 34670268 Free PMC article. Review.
-
Recent Advances in the Roles of Autophagy and Autophagy Proteins in Host Cells During Toxoplasma gondii Infection and Potential Therapeutic Implications.Front Cell Dev Biol. 2021 Jun 9;9:673813. doi: 10.3389/fcell.2021.673813. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34179003 Free PMC article. Review.
-
Host E3 ubiquitin ligase ITCH mediates Toxoplasma gondii effector GRA35-triggered NLRP1 inflammasome activation and cell-autonomous immunity.bioRxiv [Preprint]. 2023 Dec 14:2023.12.13.571530. doi: 10.1101/2023.12.13.571530. bioRxiv. 2023. Update in: mBio. 2024 Mar 13;15(3):e0330223. doi: 10.1128/mbio.03302-23. PMID: 38168400 Free PMC article. Updated. Preprint.
References
-
- Lingelbach K, Joiner KA. The parasitophorous vacuole membrane surrounding Plasmodium and Toxoplasma: an unusual compartment in infected cells. J. Cell. Sci. 1998;111(Pt 11):1467–1475. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

