Relationship between blood eosinophils, clinical characteristics, and mortality in patients with COPD
- PMID: 28694695
- PMCID: PMC5490470
- DOI: 10.2147/COPD.S129787
Relationship between blood eosinophils, clinical characteristics, and mortality in patients with COPD
Abstract
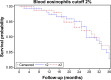
In patients with COPD, there is controversy regarding the association of blood eosinophil (Eos) levels with 1) exacerbation frequency and 2) the effect of inhaled corticosteroids for prevention of exacerbations. To determine whether Eos define subgroups of patients exhibiting attributes of COPD clinical phenotypes, we compared clinical features and mortality rates in COPD patients from the Initiatives BPCO French cohort categorized using different thresholds of blood Eos levels. The following data were collected at inclusion: medical and smoking history, occupational exposures, dyspnea, cough and sputum production, exacerbations in the previous year, history of allergy and asthma, nasal symptoms, body mass index, St George Respiratory Questionnaire (SGRQ) total score, post-bronchodilator spirometry, comorbidities, and medications. Three-year survival between groups was compared using Kaplan-Meier analysis. Three sets of analyses were performed to compare patients with ≥2% versus <2%, ≥3% versus <3%, and ≥4% versus <4% Eos. Eos was available in 458 patients (mean age: 62 years, 72% male, mean forced expiratory volume in 1 second: 51% pred), including 235 patients with Eos ≥2% (49%), 149 with Eos ≥3% (33%), and 90 with Eos ≥4% (20%). For all cutoffs, there was no difference between Eos+ and Eos- groups in univariate analyses except for diabetes and SGRQ score (more frequent and more impaired, respectively, in lower Eos categories). In particular, there was no difference in exacerbation rate, history of asthma, or three-year survival. In conclusion, regardless of the cutoff, Eos+ COPD patients exhibited no specific characteristic in terms of symptoms, lung function, exacerbation rate, and prognosis. These findings suggest that the association of higher Eos with exacerbations reported in previous studies could be population specific, which does not support generalizing the use of Eos as a biomarker for COPD phenotyping.
Keywords: COPD; eosinophils; exacerbations; quality of life; survival.
Conflict of interest statement
Disclosure Dr Zysman reports grants and personal fees from Boehringer Ingelheim and personal fees from Novartis, Chiesi, AGEvie outside the submitted work. Dr Deslee reports personal fees from Novartis, AstraZeneca, BTG/PneumRx, Chiesi, and Boehringer Ingelheim, outside the submitted work. Dr Caillaud reports grants and personal fees from Boehringer Ingelheim, personal fees from Novartis, Bayer, and grants from AstraZeneca, ALK-Abello, and Stallergenes, outside the submitted work. Dr Chanez does not report any conflicts of interest. Dr Escamilla reports grants and personal fees from Boehringer Ingelheim, Novartis, personal fees from AstraZeneca, Mundipharma, ALK, and Chiesi, and nonfinancial support from TEVA, outside the submitted work. Dr Court-Fortune reports grants and personal fees from Boehringer Ingelheim and Novartis. Dr Nesme-Meyer reports grants and personal fees from Boehringer Ingelheim. Dr Perez reports personal fees from Boehringer Ingelheim, Novartis, GSK, Chiesi, and Pierre Fabre, outside the submitted work. Dr Paillasseur reports grants from Initiative BPCO Association, during the conduct of the study. Dr Pinet reports grants and personal fees from Boehringer Ingelheim and Novartis. Dr Jebrak reports grants and personal fees from Boerhringer, Novartis, and Pfizer and personal fees from AstraZeneca during the conduct of the study. Dr Roche reports grants and personal fees from Boehringer Ingelheim, Novartis, and Pfizer and personal fees from Teva, GSK, AstraZeneca, Chiesi, Mundipharma, Cipla, Sanofi, Sandoz, Zambon, 3M, and BRAHMS/Thermo Fischer scientific, outside the submitted work. Dr Burgel reports grants and personal fees from Boehringer Ingelheim and personal fees from Aptalis, Astra-Zeneca, Chiesi, GSK, Novartis, Pfizer, Vertex, and Zambon, outside the submitted work.
Figures

Similar articles
-
Association of sputum and blood eosinophil concentrations with clinical measures of COPD severity: an analysis of the SPIROMICS cohort.Lancet Respir Med. 2017 Dec;5(12):956-967. doi: 10.1016/S2213-2600(17)30432-0. Epub 2017 Nov 13. Lancet Respir Med. 2017. PMID: 29146301 Free PMC article.
-
Relationships between symptoms and lung function in asthma and/or chronic obstructive pulmonary disease in a real-life setting: the NOVEL observational longiTudinal studY.Ther Adv Respir Dis. 2024 Jan-Dec;18:17534666241254212. doi: 10.1177/17534666241254212. Ther Adv Respir Dis. 2024. PMID: 38841799 Free PMC article.
-
Exacerbations and health care resource use among patients with COPD in relation to blood eosinophil counts.Int J Chron Obstruct Pulmon Dis. 2019 Mar 22;14:683-692. doi: 10.2147/COPD.S194367. eCollection 2019. Int J Chron Obstruct Pulmon Dis. 2019. PMID: 30962682 Free PMC article.
-
Blood eosinophil levels as a biomarker in COPD.Respir Med. 2018 May;138:21-31. doi: 10.1016/j.rmed.2018.03.016. Epub 2018 Mar 15. Respir Med. 2018. PMID: 29724389 Review.
-
Eosinophils in COPD: just another biomarker?Lancet Respir Med. 2017 Sep;5(9):747-759. doi: 10.1016/S2213-2600(17)30217-5. Epub 2017 Jun 7. Lancet Respir Med. 2017. PMID: 28601554 Review.
Cited by
-
Using Blood Eosinophil Count as a Biomarker to Guide Corticosteroid Treatment for Chronic Obstructive Pulmonary Disease.Diagnostics (Basel). 2021 Feb 3;11(2):236. doi: 10.3390/diagnostics11020236. Diagnostics (Basel). 2021. PMID: 33546498 Free PMC article. Review.
-
2017 Global Initiative for Chronic Obstructive Lung Disease reclassifies half of COPD subjects to lower risk group.Int J Chron Obstruct Pulmon Dis. 2018 Jan 3;13:165-173. doi: 10.2147/COPD.S151016. eCollection 2018. Int J Chron Obstruct Pulmon Dis. 2018. PMID: 29379281 Free PMC article.
-
Variability of blood eosinophil count and prognosis of COPD exacerbations.Ann Med. 2021 Dec;53(1):1152-1158. doi: 10.1080/07853890.2021.1949489. Ann Med. 2021. PMID: 34269633 Free PMC article.
-
Blood Eosinophil Count and Hospital Readmission in Patients with Acute Exacerbation of Chronic Obstructive Pulmonary Disease.Int J Chron Obstruct Pulmon Dis. 2020 Oct 23;15:2629-2641. doi: 10.2147/COPD.S251115. eCollection 2020. Int J Chron Obstruct Pulmon Dis. 2020. PMID: 33122901 Free PMC article.
-
From Biomarkers to Novel Therapeutic Approaches in Chronic Obstructive Pulmonary Disease.Biomedicines. 2021 Nov 8;9(11):1638. doi: 10.3390/biomedicines9111638. Biomedicines. 2021. PMID: 34829866 Free PMC article. Review.
References
-
- Vedel-Krogh S, Nielsen SF, Lange P, Vestbo J, Nordestgaard BG. Blood eosinophils and exacerbations in chronic obstructive pulmonary disease. The Copenhagen General Population Study. Am J Respir Crit Care Med. 2016;193(9):965–974. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

