Monocyte-derived alveolar macrophages drive lung fibrosis and persist in the lung over the life span
- PMID: 28694385
- PMCID: PMC5551573
- DOI: 10.1084/jem.20162152
Monocyte-derived alveolar macrophages drive lung fibrosis and persist in the lung over the life span
Abstract
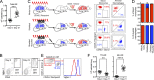
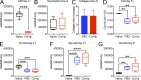
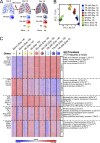
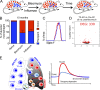
Little is known about the relative importance of monocyte and tissue-resident macrophages in the development of lung fibrosis. We show that specific genetic deletion of monocyte-derived alveolar macrophages after their recruitment to the lung ameliorated lung fibrosis, whereas tissue-resident alveolar macrophages did not contribute to fibrosis. Using transcriptomic profiling of flow-sorted cells, we found that monocyte to alveolar macrophage differentiation unfolds continuously over the course of fibrosis and its resolution. During the fibrotic phase, monocyte-derived alveolar macrophages differ significantly from tissue-resident alveolar macrophages in their expression of profibrotic genes. A population of monocyte-derived alveolar macrophages persisted in the lung for one year after the resolution of fibrosis, where they became increasingly similar to tissue-resident alveolar macrophages. Human homologues of profibrotic genes expressed by mouse monocyte-derived alveolar macrophages during fibrosis were up-regulated in human alveolar macrophages from fibrotic compared with normal lungs. Our findings suggest that selectively targeting alveolar macrophage differentiation within the lung may ameliorate fibrosis without the adverse consequences associated with global monocyte or tissue-resident alveolar macrophage depletion.
© 2017 Misharin et al.
Figures




Similar articles
-
A spatially restricted fibrotic niche in pulmonary fibrosis is sustained by M-CSF/M-CSFR signalling in monocyte-derived alveolar macrophages.Eur Respir J. 2020 Jan 16;55(1):1900646. doi: 10.1183/13993003.00646-2019. Print 2020 Jan. Eur Respir J. 2020. PMID: 31601718 Free PMC article.
-
Spatial and phenotypic heterogeneity of resident and monocyte-derived macrophages during inflammatory exacerbations leading to pulmonary fibrosis.Front Immunol. 2024 Jul 19;15:1425466. doi: 10.3389/fimmu.2024.1425466. eCollection 2024. Front Immunol. 2024. PMID: 39100672 Free PMC article.
-
Reduced supply of monocyte-derived macrophages leads to a transition from nodular to diffuse lesions and tissue cell activation in silica-induced pulmonary fibrosis in mice.Am J Pathol. 2015 Nov;185(11):2923-38. doi: 10.1016/j.ajpath.2015.07.013. Epub 2015 Oct 9. Am J Pathol. 2015. PMID: 26456580
-
Alveolar Macrophages: Adaptation to Their Anatomic Niche during and after Inflammation.Cells. 2021 Oct 12;10(10):2720. doi: 10.3390/cells10102720. Cells. 2021. PMID: 34685700 Free PMC article. Review.
-
The contributions of lung macrophage and monocyte heterogeneity to influenza pathogenesis.Immunol Cell Biol. 2017 Mar;95(3):225-235. doi: 10.1038/icb.2016.97. Epub 2016 Sep 27. Immunol Cell Biol. 2017. PMID: 27670791 Review.
Cited by
-
The role of lung macrophages in acute respiratory distress syndrome.Inflamm Res. 2022 Dec;71(12):1417-1432. doi: 10.1007/s00011-022-01645-4. Epub 2022 Oct 20. Inflamm Res. 2022. PMID: 36264361 Free PMC article. Review.
-
Alveolar macrophage metabolic programming via a C-type lectin receptor protects against lipo-toxicity and cell death.Nat Commun. 2022 Nov 25;13(1):7272. doi: 10.1038/s41467-022-34935-w. Nat Commun. 2022. PMID: 36433992 Free PMC article.
-
The Epithelial-Immune Crosstalk in Pulmonary Fibrosis.Front Immunol. 2021 May 19;12:631235. doi: 10.3389/fimmu.2021.631235. eCollection 2021. Front Immunol. 2021. PMID: 34093523 Free PMC article. Review.
-
Lung CCR6-CXCR3- type 2 helper T cells as an indicator of progressive fibrosing interstitial lung diseases.Sci Rep. 2022 Nov 15;12(1):19577. doi: 10.1038/s41598-022-24011-0. Sci Rep. 2022. PMID: 36380088 Free PMC article.
-
Proteomics reveals antiviral host response and NETosis during acute COVID-19 in high-risk patients.Biochim Biophys Acta Mol Basis Dis. 2023 Feb;1869(2):166592. doi: 10.1016/j.bbadis.2022.166592. Epub 2022 Nov 1. Biochim Biophys Acta Mol Basis Dis. 2023. PMID: 36328146 Free PMC article.
References
-
- Aschner Y., Khalifah A.P., Briones N., Yamashita C., Dolgonos L., Young S.K., Campbell M.N., Riches D.W., Redente E.F., Janssen W.J., et al. . 2014. Protein tyrosine phosphatase α mediates profibrotic signaling in lung fibroblasts through TGF-β responsiveness. Am. J. Pathol. 184:1489–1502. 10.1016/j.ajpath.2014.01.016 - DOI - PMC - PubMed
-
- Bharat A., Bhorade S.M., Morales-Nebreda L., McQuattie-Pimentel A.C., Soberanes S., Ridge K., DeCamp M.M., Mestan K.K., Perlman H., Budinger G.R., and Misharin A.V.. 2016. Flow cytometry reveals similarities between lung macrophages in humans and mice. Am. J. Respir. Cell Mol. Biol. 54:147–149. 10.1165/rcmb.2015-0147LE - DOI - PMC - PubMed
MeSH terms
Grants and funding
- R01 ES013995/ES/NIEHS NIH HHS/United States
- R37 HL048129/HL/NHLBI NIH HHS/United States
- K08 HL125940/HL/NHLBI NIH HHS/United States
- R01 HL048129/HL/NHLBI NIH HHS/United States
- R21 ES025644/ES/NIEHS NIH HHS/United States
- R56 HL127245/HL/NHLBI NIH HHS/United States
- R37 AG026647/AG/NIA NIH HHS/United States
- R03 AR061593/AR/NIAMS NIH HHS/United States
- I01 BX000201/BX/BLRD VA/United States
- K08 HL128867/HL/NHLBI NIH HHS/United States
- P01 AG049665/AG/NIA NIH HHS/United States
- P01 HL071643/HL/NHLBI NIH HHS/United States
- R01 AR064546/AR/NIAMS NIH HHS/United States
- U19 AI106683/AI/NIAID NIH HHS/United States
- UL1 TR001422/TR/NCATS NIH HHS/United States
- P30 CA060553/CA/NCI NIH HHS/United States
- R01 ES015024/ES/NIEHS NIH HHS/United States
- R01 HL128194/HL/NHLBI NIH HHS/United States
- R01 HL124664/HL/NHLBI NIH HHS/United States
- R01 HL134375/HL/NHLBI NIH HHS/United States
- R01 HL085534/HL/NHLBI NIH HHS/United States
- R01 HL079190/HL/NHLBI NIH HHS/United States
- F32 HL136111/HL/NHLBI NIH HHS/United States
- T32 DK077662/DK/NIDDK NIH HHS/United States
- K01 AR064313/AR/NIAMS NIH HHS/United States
- T32 HL076139/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

