Maintenance and Intensification of Bivalent Oral Poliovirus Vaccine Use Prior to its Coordinated Global Cessation
- PMID: 28690915
- PMCID: PMC5497833
- DOI: 10.4172/2157-7560.1000340
Maintenance and Intensification of Bivalent Oral Poliovirus Vaccine Use Prior to its Coordinated Global Cessation
Abstract
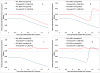
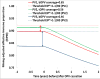
Objective: To examine the impact of different bivalent oral poliovirus vaccine (bOPV) supplemental immunization activity (SIA) strategies on population immunity to serotype 1 and 3 poliovirus transmission and circulating vaccine-derived poliovirus (cVDPV) risks before and after globally-coordinated cessation of serotype 1 and 3 oral poliovirus vaccine (OPV13 cessation).
Methods: We adapt mathematical models that previously informed vaccine choices ahead of the trivalent oral poliovirus vaccine to bOPV switch to estimate the population immunity to serotype 1 and 3 poliovirus transmission needed at the time of OPV13 cessation to prevent subsequent cVDPV outbreaks. We then examine the impact of different frequencies of SIAs using bOPV in high risk populations on population immunity to serotype 1 and 3 transmission, on the risk of serotype 1 and 3 cVDPV outbreaks, and on the vulnerability to any imported bOPV-related polioviruses.
Results: Maintaining high population immunity to serotype 1 and 3 transmission using bOPV SIAs significantly reduces 1) the risk of outbreaks due to imported serotype 1 and 3 viruses, 2) the emergence of indigenous cVDPVs before or after OPV13 cessation, and 3) the vulnerability to bOPV-related polioviruses in the event of non-synchronous OPV13 cessation or inadvertent bOPV use after OPV13 cessation.
Conclusion: Although some reduction in global SIA frequency can safely occur, countries with suboptimal routine immunization coverage should each continue to conduct at least one annual SIA with bOPV, preferably more, until global OPV13 cessation. Preventing cVDPV risks after OPV13 cessation requires investments in bOPV SIAs now through the time of OPV13 cessation.
Keywords: Eradication; OPV; Polio; Risk management; Vaccine choice.
Conflict of interest statement
Competing Interests None
Figures

Similar articles
-
Coordinated global cessation of oral poliovirus vaccine use: Options and potential consequences.Risk Anal. 2024 Feb;44(2):366-378. doi: 10.1111/risa.14158. Epub 2023 Jun 21. Risk Anal. 2024. PMID: 37344934 Free PMC article. Review.
-
Implementation of coordinated global serotype 2 oral poliovirus vaccine cessation: risks of potential non-synchronous cessation.BMC Infect Dis. 2016 May 26;16:231. doi: 10.1186/s12879-016-1536-9. BMC Infect Dis. 2016. PMID: 27230071 Free PMC article.
-
Managing the risk of circulating vaccine-derived poliovirus during the endgame: oral poliovirus vaccine needs.BMC Infect Dis. 2015 Sep 24;15:390. doi: 10.1186/s12879-015-1114-6. BMC Infect Dis. 2015. PMID: 26404780 Free PMC article.
-
The differential impact of oral poliovirus vaccine formulation choices on serotype-specific population immunity to poliovirus transmission.BMC Infect Dis. 2015 Sep 17;15:376. doi: 10.1186/s12879-015-1116-4. BMC Infect Dis. 2015. PMID: 26382234 Free PMC article.
-
Circulating vaccine-derived polioviruses: current state of knowledge.Bull World Health Organ. 2004 Jan;82(1):16-23. Epub 2004 Feb 26. Bull World Health Organ. 2004. PMID: 15106296 Free PMC article. Review.
Cited by
-
Increasing Population Immunity Prior to Globally-Coordinated Cessation of Bivalent Oral Poliovirus Vaccine (bOPV).Pathogens. 2024 Sep 17;13(9):804. doi: 10.3390/pathogens13090804. Pathogens. 2024. PMID: 39338995 Free PMC article.
-
Health Economic Analysis of Antiviral Drugs in the Global Polio Eradication Endgame.Med Decis Making. 2023 Oct-Nov;43(7-8):850-862. doi: 10.1177/0272989X231191127. Epub 2023 Aug 14. Med Decis Making. 2023. PMID: 37577803 Free PMC article.
-
Complexity of options related to restarting oral poliovirus vaccine (OPV) in national immunization programs after OPV cessation.Gates Open Res. 2023 Apr 17;7:55. doi: 10.12688/gatesopenres.14511.1. eCollection 2023. Gates Open Res. 2023. PMID: 37547300 Free PMC article.
-
Health effects of routine measles vaccination and supplementary immunisation activities in 14 high-burden countries: a Dynamic Measles Immunization Calculation Engine (DynaMICE) modelling study.Lancet Glob Health. 2023 Aug;11(8):e1194-e1204. doi: 10.1016/S2214-109X(23)00220-6. Lancet Glob Health. 2023. PMID: 37474227 Free PMC article.
-
Coordinated global cessation of oral poliovirus vaccine use: Options and potential consequences.Risk Anal. 2024 Feb;44(2):366-378. doi: 10.1111/risa.14158. Epub 2023 Jun 21. Risk Anal. 2024. PMID: 37344934 Free PMC article. Review.
References
-
- World Health Organization. Transmission of wild poliovirus type 2 -Apparent global interruption. Wkly Epidemiol Rec. 2001;76:95–97. - PubMed
-
- Global Polio Eradication Initiative. Global eradication of wild poliovirus type 2 declared 2015
-
- World Health Organization. Polio Eradication Initiative. Cessation of Routine Oral Polio Vaccine (OPV) use after Global Polio Eradication. Framework for National Policy Makers in OPV-using Countries 2005
-
- Duintjer Tebbens RJ, Pallansch MA, Kew OM, Cáceres VM, Jafari H, et al. Risks of paralytic disease due to wild or vaccine-derived poliovirus after eradication. Risk Anal. 2006;26:1471–1505. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
