Metabolic Products of Linalool and Modulation of GABAA Receptors
- PMID: 28680877
- PMCID: PMC5478857
- DOI: 10.3389/fchem.2017.00046
Metabolic Products of Linalool and Modulation of GABAA Receptors
Abstract
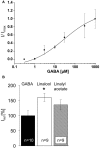
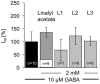
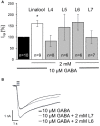
Terpenoids are major subcomponents in aroma substances which harbor sedative physiological potential. We have demonstrated that various monoterpenoids such as the acyclic linalool enhance GABAergic currents in an allosteric manner in vitro upon overexpression of inhibitory α1β2 GABAA receptors in various expression systems. However, in plants or humans, i.e., following intake via inhalation or ingestion, linalool undergoes metabolic modifications including oxygenation and acetylation, which may affect the modulatory efficacy of the generated linalool derivatives. Here, we analyzed the modulatory potential of linalool derivatives at α1β2γ2 GABAA receptors upon transient overexpression. Following receptor expression control, electrophysiological recordings in a whole cell configuration were used to determine the chloride influx upon co-application of GABA EC10-30 together with the modulatory substance. Our results show that only oxygenated linalool metabolites at carbon 8 positively affect GABAergic currents whereas derivatives hydroxylated or carboxylated at carbon 8 were rather ineffective. Acetylated linalool derivatives resulted in non-significant changes of GABAergic currents. We can conclude that metabolism of linalool reduces its positive allosteric potential at GABAA receptors compared to the significant potentiation effects of the parent molecule linalool itself.
Keywords: Cys-loop receptor; GABAA receptor; linalool; linalyl acetate; oxygenation; patch-clamp.
Figures
Similar articles
-
The terpenoids Myrtenol and Verbenol act on δ subunit-containing GABAA receptors and enhance tonic inhibition in dentate gyrus granule cells.Neurosci Lett. 2016 Aug 15;628:91-7. doi: 10.1016/j.neulet.2016.06.027. Epub 2016 Jun 14. Neurosci Lett. 2016. PMID: 27312536
-
The dual modulatory effects of efavirenz on GABAA receptors are mediated via two distinct sites.Neuropharmacology. 2017 Jul 15;121:167-178. doi: 10.1016/j.neuropharm.2017.04.038. Epub 2017 Apr 27. Neuropharmacology. 2017. PMID: 28456686 Free PMC article.
-
Structural changes at the myrtenol backbone reverse its positive allosteric potential into inhibitory GABAA receptor modulation.Biol Chem. 2018 May 24;399(6):549-563. doi: 10.1515/hsz-2017-0262. Biol Chem. 2018. PMID: 29408795
-
Structure-odor relationships of linalool, linalyl acetate and their corresponding oxygenated derivatives.Front Chem. 2015 Oct 6;3:57. doi: 10.3389/fchem.2015.00057. eCollection 2015. Front Chem. 2015. PMID: 26501053 Free PMC article.
-
Post-episode depression of GABAergic transmission in spinal neurons of the chick embryo.J Neurophysiol. 2001 May;85(5):2166-76. doi: 10.1152/jn.2001.85.5.2166. J Neurophysiol. 2001. PMID: 11353031
Cited by
-
Effect of Lavender Oil Leg Massage on Physical, Cognitive, and Psychological Variables of Patients with Hypertension: A Randomized Controlled Trial.Int J Ther Massage Bodywork. 2024 Sep 12;17(3):15-22. doi: 10.3822/ijtmb.v17i3.897. eCollection 2024 Sep. Int J Ther Massage Bodywork. 2024. PMID: 39267900 Free PMC article.
-
Maternal linalool treatment protects against radiofrequency wave-induced deteriorations in adolescent rats: A behavioral and electrophysiological study.Sci Rep. 2024 Jul 27;14(1):17257. doi: 10.1038/s41598-024-68103-5. Sci Rep. 2024. PMID: 39060318 Free PMC article.
-
Timut Pepper Extract Slows Age-Dependent Decline of Mobility and Collagen Loss and Promotes Longevity.Nutrients. 2024 Jul 3;16(13):2122. doi: 10.3390/nu16132122. Nutrients. 2024. PMID: 38999870 Free PMC article.
-
Antimicrobial, Probiotic, and Immunomodulatory Potential of Cannabis sativa Extract and Delivery Systems.Antibiotics (Basel). 2024 Apr 17;13(4):369. doi: 10.3390/antibiotics13040369. Antibiotics (Basel). 2024. PMID: 38667045 Free PMC article.
-
Aromatherapy for the brain: Lavender's healing effect on epilepsy, depression, anxiety, migraine, and Alzheimer's disease: A review article.Heliyon. 2023 Jul 20;9(8):e18492. doi: 10.1016/j.heliyon.2023.e18492. eCollection 2023 Aug. Heliyon. 2023. PMID: 37554839 Free PMC article. Review.
References
-
- Aprotosoaie A. C., Hãncianu M., Costache I. I., Miron A. (2014). Linalool: a review on a key odorant molecule with valuable biological properties. Flavour Frag. J. 29, 193–219. 10.1002/ffj.3197 - DOI
-
- Boachon B., Junker R. R., Miesch L., Bassard J. E., Hofer R., Caillieaudeaux R., et al. . (2015). CYP76C1 (Cytochrome P450)-mediated linalool metabolism and the formation of volatile and soluble linalool oxides in Arabidopsis flowers: a strategy for defense against floral antagonists. Plant Cell 27, 2972–2990. 10.1105/tpc.15.00399 - DOI - PMC - PubMed
-
- Carrasco A., Perez E., Cutillas A. B., Martinez-Gutierrez R., Tomas V., Tudela J. (2016). Origanum vulgare and thymbra capitata essential oils from spain: determination of aromatic profile and bioactivities. Nat. Prod. Commun. 11, 113–120. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

