The Role of the Multifunctional BAG3 Protein in Cellular Protein Quality Control and in Disease
- PMID: 28680391
- PMCID: PMC5478690
- DOI: 10.3389/fnmol.2017.00177
The Role of the Multifunctional BAG3 Protein in Cellular Protein Quality Control and in Disease
Abstract
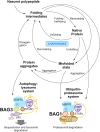
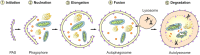
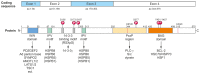
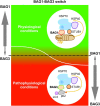
In neurons, but also in all other cells the complex proteostasis network is monitored and tightly regulated by the cellular protein quality control (PQC) system. Beyond folding of newly synthesized polypeptides and their refolding upon misfolding the PQC also manages the disposal of aberrant proteins either by the ubiquitin-proteasome machinery or by the autophagic-lysosomal system. Aggregated proteins are primarily degraded by a process termed selective macroautophagy (or aggrephagy). One such recently discovered selective macroautophagy pathway is mediated by the multifunctional HSP70 co-chaperone BAG3 (BCL-2-associated athanogene 3). Under acute stress and during cellular aging, BAG3 in concert with the molecular chaperones HSP70 and HSPB8 as well as the ubiquitin receptor p62/SQSTM1 specifically targets aggregation-prone proteins to autophagic degradation. Thereby, BAG3-mediated selective macroautophagy represents a pivotal adaptive safeguarding and emergency system of the PQC which is activated under pathophysiological conditions to ensure cellular proteostasis. Interestingly, BAG3-mediated selective macroautophagy is also involved in the clearance of aggregated proteins associated with age-related neurodegenerative disorders, like Alzheimer's disease (tau-protein), Huntington's disease (mutated huntingtin/polyQ proteins), and amyotrophic lateral sclerosis (mutated SOD1). In addition, based on its initial description BAG3 is an anti-apoptotic protein that plays a decisive role in other widespread diseases, including cancer and myopathies. Therefore, in the search for novel therapeutic intervention avenues in neurodegeneration, myopathies and cancer BAG3 is a promising candidate.
Keywords: BAG3; neurodegenerative disorders; protein quality control; proteostasis; selective macroautophagy.
Figures

Similar articles
-
BAG3 Proteomic Signature under Proteostasis Stress.Cells. 2020 Nov 4;9(11):2416. doi: 10.3390/cells9112416. Cells. 2020. PMID: 33158300 Free PMC article.
-
The chaperone-assisted selective autophagy complex dynamics and dysfunctions.Autophagy. 2023 Jun;19(6):1619-1641. doi: 10.1080/15548627.2022.2160564. Epub 2023 Jan 3. Autophagy. 2023. PMID: 36594740 Free PMC article.
-
Protein Quality Control by Molecular Chaperones in Neurodegeneration.Front Neurosci. 2017 Apr 6;11:185. doi: 10.3389/fnins.2017.00185. eCollection 2017. Front Neurosci. 2017. PMID: 28428740 Free PMC article. Review.
-
Enhanced autophagic-lysosomal activity and increased BAG3-mediated selective macroautophagy as adaptive response of neuronal cells to chronic oxidative stress.Redox Biol. 2019 Jun;24:101181. doi: 10.1016/j.redox.2019.101181. Epub 2019 Apr 2. Redox Biol. 2019. PMID: 30959460 Free PMC article.
-
HspB8 and Bag3: a new chaperone complex targeting misfolded proteins to macroautophagy.Autophagy. 2008 Feb;4(2):237-9. doi: 10.4161/auto.5407. Epub 2007 Dec 11. Autophagy. 2008. PMID: 18094623 Review.
Cited by
-
The role of BAG3 in health and disease: A "Magic BAG of Tricks".J Cell Biochem. 2022 Jan;123(1):4-21. doi: 10.1002/jcb.29952. Epub 2021 May 14. J Cell Biochem. 2022. PMID: 33987872 Free PMC article. Review.
-
Phytol and α-Bisabolol Synergy Induces Autophagy and Apoptosis in A549 Cells and Additional Molecular Insights through Comprehensive Proteome Analysis via Nano LC-MS/MS.Anticancer Agents Med Chem. 2024;24(10):773-788. doi: 10.2174/0118715206289038240214102951. Anticancer Agents Med Chem. 2024. PMID: 38415491
-
Neuroprotection by Heat Shock Factor-1 (HSF1) and Trimerization-Deficient Mutant Identifies Novel Alterations in Gene Expression.Sci Rep. 2018 Nov 22;8(1):17255. doi: 10.1038/s41598-018-35610-1. Sci Rep. 2018. PMID: 30467350 Free PMC article.
-
Drosophila NUAK functions with Starvin/BAG3 in autophagic protein turnover.PLoS Genet. 2020 Apr 22;16(4):e1008700. doi: 10.1371/journal.pgen.1008700. eCollection 2020 Apr. PLoS Genet. 2020. PMID: 32320396 Free PMC article.
-
Neuromuscular Diseases Due to Chaperone Mutations: A Review and Some New Results.Int J Mol Sci. 2020 Feb 19;21(4):1409. doi: 10.3390/ijms21041409. Int J Mol Sci. 2020. PMID: 32093037 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

