Dynamin-2 mutations linked to Centronuclear Myopathy impair actin-dependent trafficking in muscle cells
- PMID: 28676641
- PMCID: PMC5496902
- DOI: 10.1038/s41598-017-04418-w
Dynamin-2 mutations linked to Centronuclear Myopathy impair actin-dependent trafficking in muscle cells
Abstract
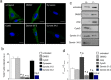
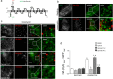
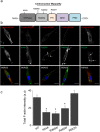
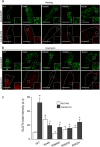
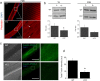
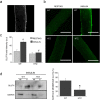
Dynamin-2 is a ubiquitously expressed GTP-ase that mediates membrane remodeling. Recent findings indicate that dynamin-2 also regulates actin dynamics. Mutations in dynamin-2 cause dominant centronuclear myopathy (CNM), a congenital myopathy characterized by progressive weakness and atrophy of skeletal muscles. However, the muscle-specific roles of dynamin-2 affected by these mutations remain elusive. Here we show that, in muscle cells, the GTP-ase activity of dynamin-2 is involved in de novo actin polymerization as well as in actin-mediated trafficking of the glucose transporter GLUT4. Expression of dynamin-2 constructs carrying CNM-linked mutations disrupted the formation of new actin filaments as well as the stimulus-induced translocation of GLUT4 to the plasma membrane. Similarly, mature muscle fibers isolated from heterozygous knock-in mice that harbor the dynamin-2 mutation p.R465W, an animal model of CNM, exhibited altered actin organization, reduced actin polymerization and impaired insulin-induced translocation of GLUT4 to the sarcolemma. Moreover, GLUT4 displayed aberrant perinuclear accumulation in biopsies from CNM patients carrying dynamin-2 mutations, further suggesting trafficking defects. These results suggest that dynamin-2 is a key regulator of actin dynamics and GLUT4 trafficking in muscle cells. Our findings also support a model in which impairment of actin-dependent trafficking contributes to the pathological mechanism in dynamin-2-associated CNM.
Conflict of interest statement
P.C. declares IP protection on the RCMH cell line. All other authors have no competing financial interests to declare.
Figures

Similar articles
-
A centronuclear myopathy-dynamin 2 mutation impairs skeletal muscle structure and function in mice.Hum Mol Genet. 2010 Dec 15;19(24):4820-36. doi: 10.1093/hmg/ddq413. Epub 2010 Sep 21. Hum Mol Genet. 2010. PMID: 20858595
-
Dynamin-2 mutations linked to neonatal-onset centronuclear myopathy impair exocytosis and endocytosis in adrenal chromaffin cells.J Neurochem. 2024 Sep;168(9):3268-3283. doi: 10.1111/jnc.16194. Epub 2024 Aug 10. J Neurochem. 2024. PMID: 39126680
-
Gain-of-Function Dynamin-2 Mutations Linked to Centronuclear Myopathy Impair Ca2+-Induced Exocytosis in Human Myoblasts.Int J Mol Sci. 2022 Sep 8;23(18):10363. doi: 10.3390/ijms231810363. Int J Mol Sci. 2022. PMID: 36142275 Free PMC article.
-
Structural insights into the centronuclear myopathy-associated functions of BIN1 and dynamin 2.J Struct Biol. 2016 Oct;196(1):37-47. doi: 10.1016/j.jsb.2016.06.015. Epub 2016 Jun 23. J Struct Biol. 2016. PMID: 27343996 Free PMC article. Review.
-
Dynamin 2-related centronuclear myopathy: clinical, histological and genetic aspects of further patients and review of the literature.Clin Neuropathol. 2008 Nov-Dec;27(6):430-8. doi: 10.5414/npp27430. Clin Neuropathol. 2008. PMID: 19130742 Review.
Cited by
-
EHBP1L1 Frameshift Deletion in English Springer Spaniel Dogs with Dyserythropoietic Anemia and Myopathy Syndrome (DAMS) or Neonatal Losses.Genes (Basel). 2022 Aug 26;13(9):1533. doi: 10.3390/genes13091533. Genes (Basel). 2022. PMID: 36140701 Free PMC article.
-
Structural Insights into the Mechanism of Dynamin Superfamily Proteins.Trends Cell Biol. 2019 Mar;29(3):257-273. doi: 10.1016/j.tcb.2018.11.003. Epub 2018 Dec 5. Trends Cell Biol. 2019. PMID: 30527453 Free PMC article. Review.
-
Nuclear defects in skeletal muscle from a Dynamin 2-linked centronuclear myopathy mouse model.Sci Rep. 2019 Feb 7;9(1):1580. doi: 10.1038/s41598-018-38184-0. Sci Rep. 2019. PMID: 30733559 Free PMC article.
-
N-Acetylcysteine Reduces Skeletal Muscles Oxidative Stress and Improves Grip Strength in Dysferlin-Deficient Bla/J Mice.Int J Mol Sci. 2020 Jun 16;21(12):4293. doi: 10.3390/ijms21124293. Int J Mol Sci. 2020. PMID: 32560255 Free PMC article.
-
Congenital myopathies: pathophysiological mechanisms and promising therapies.J Transl Med. 2024 Sep 2;22(1):815. doi: 10.1186/s12967-024-05626-5. J Transl Med. 2024. PMID: 39223631 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

