Synergistic effects of treating the spinal cord and brain in CLN1 disease
- PMID: 28673981
- PMCID: PMC5530669
- DOI: 10.1073/pnas.1701832114
Synergistic effects of treating the spinal cord and brain in CLN1 disease
Abstract
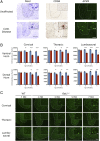
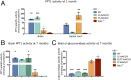
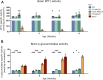
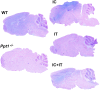
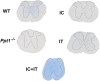
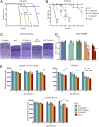
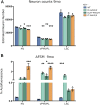
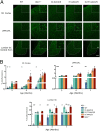
Infantile neuronal ceroid lipofuscinosis (INCL, or CLN1 disease) is an inherited neurodegenerative storage disorder caused by a deficiency of the lysosomal enzyme palmitoyl protein thioesterase 1 (PPT1). It was widely believed that the pathology associated with INCL was limited to the brain, but we have now found unexpectedly profound pathology in the human INCL spinal cord. Similar pathological changes also occur at every level of the spinal cord of PPT1-deficient (Ppt1-/- ) mice before the onset of neuropathology in the brain. Various forebrain-directed gene therapy approaches have only had limited success in Ppt1-/- mice. Targeting the spinal cord via intrathecal administration of an adeno-associated virus (AAV) gene transfer vector significantly prevented pathology and produced significant improvements in life span and motor function in Ppt1-/- mice. Surprisingly, forebrain-directed gene therapy resulted in essentially no PPT1 activity in the spinal cord, and vice versa. This leads to a reciprocal pattern of histological correction in the respective tissues when comparing intracranial with intrathecal injections. However, the characteristic pathological features of INCL were almost completely absent in both the brain and spinal cord when intracranial and intrathecal injections of the same AAV vector were combined. Targeting both the brain and spinal cord also produced dramatic and synergistic improvements in motor function with an unprecedented increase in life span. These data show that spinal cord pathology significantly contributes to the clinical progression of INCL and can be effectively targeted therapeutically. This has important implications for the delivery of therapies in INCL, and potentially in other similar disorders.
Keywords: adeno-associated virus; brain; combination therapy; infantile Batten disease; spinal cord.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Intrathecal enzyme replacement therapy improves motor function and survival in a preclinical mouse model of infantile neuronal ceroid lipofuscinosis.Mol Genet Metab. 2015 Sep-Oct;116(1-2):98-105. doi: 10.1016/j.ymgme.2015.05.005. Epub 2015 May 12. Mol Genet Metab. 2015. PMID: 25982063
-
Adeno-associated virus 2-mediated gene therapy decreases autofluorescent storage material and increases brain mass in a murine model of infantile neuronal ceroid lipofuscinosis.Neurobiol Dis. 2004 Jul;16(2):360-9. doi: 10.1016/j.nbd.2004.03.005. Neurobiol Dis. 2004. PMID: 15193292
-
Synergistic effects of central nervous system-directed gene therapy and bone marrow transplantation in the murine model of infantile neuronal ceroid lipofuscinosis.Ann Neurol. 2012 Jun;71(6):797-804. doi: 10.1002/ana.23545. Epub 2012 Feb 24. Ann Neurol. 2012. PMID: 22368049 Free PMC article.
-
Pathogenesis and therapies for infantile neuronal ceroid lipofuscinosis (infantile CLN1 disease).Biochim Biophys Acta. 2013 Nov;1832(11):1906-9. doi: 10.1016/j.bbadis.2013.05.026. Epub 2013 Jun 6. Biochim Biophys Acta. 2013. PMID: 23747979 Free PMC article. Review.
-
CLN2 Disease (Classic Late Infantile Neuronal Ceroid Lipofuscinosis).Pediatr Endocrinol Rev. 2016 Jun;13 Suppl 1:682-8. Pediatr Endocrinol Rev. 2016. PMID: 27491216 Review.
Cited by
-
A Novel Porcine Model of CLN2 Batten Disease that Recapitulates Patient Phenotypes.Neurotherapeutics. 2022 Oct;19(6):1905-1919. doi: 10.1007/s13311-022-01296-7. Epub 2022 Sep 13. Neurotherapeutics. 2022. PMID: 36100791 Free PMC article.
-
Neuronal ceroid lipofuscinosis: genetic and phenotypic spectrum of 14 patients from Turkey.Neurol Sci. 2021 Mar;42(3):1103-1111. doi: 10.1007/s10072-021-05067-8. Epub 2021 Jan 23. Neurol Sci. 2021. PMID: 33486620
-
An innovative hematopoietic stem cell gene therapy approach benefits CLN1 disease in the mouse model.EMBO Mol Med. 2023 Apr 11;15(4):e15968. doi: 10.15252/emmm.202215968. Epub 2023 Mar 6. EMBO Mol Med. 2023. PMID: 36876653 Free PMC article.
-
Batten disease: an expert update on agents in preclinical and clinical trials.Expert Opin Investig Drugs. 2020 Dec;29(12):1317-1322. doi: 10.1080/13543784.2020.1837110. Epub 2020 Nov 1. Expert Opin Investig Drugs. 2020. PMID: 33135495 Free PMC article. No abstract available.
-
Depalmitoylation by Palmitoyl-Protein Thioesterase 1 in Neuronal Health and Degeneration.Front Synaptic Neurosci. 2019 Aug 29;11:25. doi: 10.3389/fnsyn.2019.00025. eCollection 2019. Front Synaptic Neurosci. 2019. PMID: 31555119 Free PMC article. Review.
References
-
- Jalanko A, Braulke T. Neuronal ceroid lipofuscinoses. Biochim Biophys Acta. 2009;1793:697–709. - PubMed
-
- Warrier V, Vieira M, Mole SE. Genetic basis and phenotypic correlations of the neuronal ceroid lipofusinoses. Biochim Biophys Acta. 2013;1832:1827–1830. - PubMed
-
- Santavuori P, Haltia M, Rapola J. Infantile type of so-called neuronal ceroid-lipofuscinosis. Dev Med Child Neurol. 1974;16:644–653. - PubMed
-
- Vesa J, et al. Mutations in the palmitoyl protein thioesterase gene causing infantile neuronal ceroid lipofuscinosis. Nature. 1995;376:584–587. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

