Phase I study of salazosulfapyridine in combination with cisplatin and pemetrexed for advanced non-small-cell lung cancer
- PMID: 28667792
- PMCID: PMC5581516
- DOI: 10.1111/cas.13309
Phase I study of salazosulfapyridine in combination with cisplatin and pemetrexed for advanced non-small-cell lung cancer
Abstract
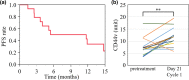
Spliced variant isoforms of CD44 (CD44v) are a marker of cancer stem cells in solid tumors. They stabilize the xCT subunit of the transporter system xc(-) and thereby promote synthesis of the antioxidant glutathione. Salazosulfapyridine (SASP) is an inhibitor of xCT and suppresses the proliferation of CD44v-positive cancer cells. Chemotherapy-naïve patients with advanced non-squamous non-small-cell lung cancer were enrolled in a dose-escalation study (standard 3 + 3 design) of SASP in combination with cisplatin and pemetrexed. The primary end-point was the percentage of patients who experience dose-limiting toxicity. Fifteen patients were enrolled in the study. Dose-limiting toxicity was observed in one of six patients at a SASP dose of 1.5 g/day (elevation of aspartate and alanine aminotransferase levels, each of grade 3), two of five patients at 3 g/day (hypotension or pneumonitis, each of grade 3), and two of three patients at 4.5 g/day (anorexia of grade 3). The maximum tolerated dose was thus 3 g/day, and the recommended dose was 1.5 g/day. The overall response rate was 26.7% and median progression-free survival was 11.7 months, much longer than that for cisplatin-pemetrexed alone in previous studies. Exposure to SASP varied markedly among individuals according to ABCG2 and NAT2 genotypes. The serum concentration of free CD44v protein was increased after the first cycle of treatment, possibly reflecting death of cancer stem cells. Salazosulfapyridine was thus given safely in combination with cisplatin-pemetrexed, with the addition of SASP tending to prolong progression-free survival. This trial is registered in the UMIN Clinical Trials Registry as UMIN000017854.
Keywords: CD44v; Cancer stem cell; non-small-cell lung cancer; oxidative stress; salazosulfapyridine.
© 2017 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Figures
Similar articles
-
Maintenance therapy with pemetrexed plus best supportive care versus placebo plus best supportive care after induction therapy with pemetrexed plus cisplatin for advanced non-squamous non-small-cell lung cancer (PARAMOUNT): a double-blind, phase 3, randomised controlled trial.Lancet Oncol. 2012 Mar;13(3):247-55. doi: 10.1016/S1470-2045(12)70063-3. Epub 2012 Feb 16. Lancet Oncol. 2012. PMID: 22341744 Clinical Trial.
-
Phase 1 study of sulfasalazine and cisplatin for patients with CD44v-positive gastric cancer refractory to cisplatin (EPOC1407).Gastric Cancer. 2017 Nov;20(6):1004-1009. doi: 10.1007/s10120-017-0720-y. Epub 2017 May 2. Gastric Cancer. 2017. PMID: 28466360 Clinical Trial.
-
A phase IB dose-escalation study of the safety and pharmacokinetics of pictilisib in combination with either paclitaxel and carboplatin (with or without bevacizumab) or pemetrexed and cisplatin (with or without bevacizumab) in patients with advanced non-small cell lung cancer.Eur J Cancer. 2017 Nov;86:186-196. doi: 10.1016/j.ejca.2017.08.027. Epub 2017 Oct 6. Eur J Cancer. 2017. PMID: 28992562 Clinical Trial.
-
Salvage treatment with irinotecan/cisplatin versus pemetrexed/cisplatin in patients with non-small cell lung cancer pre-treated with a non-platinum-based regimen in the first-line setting: a randomized phase II study of the Hellenic Oncology Research Group (HORG).Clin Transl Oncol. 2017 Mar;19(3):317-325. doi: 10.1007/s12094-016-1532-y. Epub 2016 Aug 4. Clin Transl Oncol. 2017. PMID: 27492015 Clinical Trial.
-
Canadian Cancer Trials Group (CCTG) IND215: A phase Ib study of Selumetinib in patients with untreated advanced or metastatic NSCLC who are receiving standard chemotherapy regimens.Invest New Drugs. 2019 Jun;37(3):498-506. doi: 10.1007/s10637-018-0680-z. Epub 2018 Oct 13. Invest New Drugs. 2019. PMID: 30317534 Clinical Trial.
Cited by
-
Impact of the glutathione synthesis pathway on sulfasalazine-treated endometrial cancer.Oncotarget. 2022 Jan 26;13:224-236. doi: 10.18632/oncotarget.28185. eCollection 2022. Oncotarget. 2022. PMID: 35106124 Free PMC article.
-
Oral Conventional Synthetic Disease-Modifying Antirheumatic Drugs with Antineoplastic Potential: a Review.Dermatol Ther (Heidelb). 2022 Apr;12(4):835-860. doi: 10.1007/s13555-022-00713-1. Epub 2022 Apr 5. Dermatol Ther (Heidelb). 2022. PMID: 35381976 Free PMC article. Review.
-
Can drug repurposing stop "chase and run" between aldehydes and reactive sulfur species in anti-cancer therapy?Oncotarget. 2018 Oct 2;9(77):34453-34454. doi: 10.18632/oncotarget.26170. eCollection 2018 Oct 2. Oncotarget. 2018. PMID: 30349638 Free PMC article. No abstract available.
-
Mechanism of Cell Death by Combined Treatment with an xCT Inhibitor and Paclitaxel: An Alternative Therapeutic Strategy for Patients with Ovarian Clear Cell Carcinoma.Int J Mol Sci. 2023 Jul 22;24(14):11781. doi: 10.3390/ijms241411781. Int J Mol Sci. 2023. PMID: 37511540 Free PMC article.
-
Establishment of a New Canine Inflammatory Mammary Carcinoma Cell Line and Analysis of its Cystine-glutamate Transporter Subunit Expression.J Vet Res. 2022 May 31;66(2):273-279. doi: 10.2478/jvetres-2022-0023. eCollection 2022 Jun. J Vet Res. 2022. PMID: 35892110 Free PMC article.
References
-
- Clevers H. The cancer stem cell: premises, promises and challenges. Nat Med 2011; 17: 313–19. - PubMed
-
- Trachootham D, Alexandre J, Huang P. Targeting cancer cells by ROS‐mediated mechanisms: a radical therapeutic approach? Nat Rev Drug Discov 2009; 8: 579–91. - PubMed
-
- Nagano O, Okazaki S, Saya H. Redox regulation in stem‐like cancer cells by CD44 variant isoforms. Oncogene 2013; 32: 5191–8. - PubMed
-
- Ponta H, Sherman L, Herrlich PA. CD44: from adhesion molecules to signalling regulators. Nat Rev Mol Cell Biol 2003; 4: 33–45. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

