A New AAV10-U7-Mediated Gene Therapy Prolongs Survival and Restores Function in an ALS Mouse Model
- PMID: 28663100
- PMCID: PMC5589057
- DOI: 10.1016/j.ymthe.2017.05.017
A New AAV10-U7-Mediated Gene Therapy Prolongs Survival and Restores Function in an ALS Mouse Model
Abstract
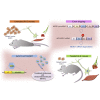
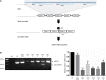
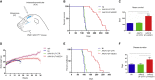
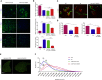
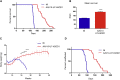
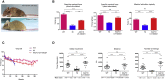
One of the most promising therapeutic approaches for familial amyotrophic lateral sclerosis linked to superoxide dismutase 1 (SOD1) is the suppression of toxic mutant SOD1 in the affected tissues. Here, we report an innovative molecular strategy for inducing substantial, widespread, and sustained reduction of mutant human SOD1 (hSOD1) levels throughout the body of SOD1G93A mice, leading to therapeutic effects in animals. Adeno-associated virus serotype rh10 vectors (AAV10) were used to mediate exon skipping of the hSOD1 pre-mRNA by expression of exon-2-targeted antisense sequences embedded in a modified U7 small-nuclear RNA (AAV10-U7-hSOD). Skipping of hSOD1 exon 2 led to the generation of a premature termination codon, inducing production of a deleted transcript that was subsequently degraded by the activation of nonsense-mediated decay. Combined intravenous and intracerebroventricular delivery of AAV10-U7-hSOD increased the survival of SOD1G93A mice injected either at birth or at 50 days of age (by 92% and 58%, respectively) and prevented weight loss and the decline of neuromuscular function. This study reports the effectiveness of an exon-skipping approach in SOD1-ALS mice, supporting the translation of this technology to the treatment of this as yet incurable disease.
Keywords: AAV; ALS; exon skipping; gene therapy; hSOD1.
Copyright © 2017 The American Society of Gene and Cell Therapy. Published by Elsevier Inc. All rights reserved.
Figures

Similar articles
-
AAV2/9-mediated overexpression of MIF inhibits SOD1 misfolding, delays disease onset, and extends survival in mouse models of ALS.Proc Natl Acad Sci U S A. 2019 Jul 16;116(29):14755-14760. doi: 10.1073/pnas.1904665116. Epub 2019 Jul 1. Proc Natl Acad Sci U S A. 2019. PMID: 31262807 Free PMC article.
-
Treatment of a Mouse Model of ALS by In Vivo Base Editing.Mol Ther. 2020 Apr 8;28(4):1177-1189. doi: 10.1016/j.ymthe.2020.01.005. Epub 2020 Jan 14. Mol Ther. 2020. PMID: 31991108 Free PMC article.
-
Adeno-associated virus-delivered artificial microRNA extends survival and delays paralysis in an amyotrophic lateral sclerosis mouse model.Ann Neurol. 2016 Apr;79(4):687-700. doi: 10.1002/ana.24618. Epub 2016 Mar 11. Ann Neurol. 2016. PMID: 26891182 Free PMC article.
-
Silence superoxide dismutase 1 (SOD1): a promising therapeutic target for amyotrophic lateral sclerosis (ALS).Expert Opin Ther Targets. 2020 Apr;24(4):295-310. doi: 10.1080/14728222.2020.1738390. Epub 2020 Mar 14. Expert Opin Ther Targets. 2020. PMID: 32125907 Review.
-
Silencing strategies for therapy of SOD1-mediated ALS.Neurosci Lett. 2017 Jan 1;636:32-39. doi: 10.1016/j.neulet.2016.07.059. Epub 2016 Aug 6. Neurosci Lett. 2017. PMID: 27507699 Review.
Cited by
-
Amyotrophic Lateral Sclerosis: Insights and New Prospects in Disease Pathophysiology, Biomarkers and Therapies.Pharmaceuticals (Basel). 2024 Oct 18;17(10):1391. doi: 10.3390/ph17101391. Pharmaceuticals (Basel). 2024. PMID: 39459030 Free PMC article. Review.
-
Combination AAV therapy with galectin-1 and SOD1 downregulation demonstrates superior therapeutic effect in a severe ALS mouse model.Mol Ther Methods Clin Dev. 2024 Aug 6;32(3):101312. doi: 10.1016/j.omtm.2024.101312. eCollection 2024 Sep 12. Mol Ther Methods Clin Dev. 2024. PMID: 39257530 Free PMC article.
-
The Efficacy and Safety of Intrathecal Autologous Bone Marrow-Derived Mesenchymal Stromal Cells in Amyotrophic Lateral Sclerosis: A Pilot Study.Adv Pharm Bull. 2023 Mar;13(2):361-367. doi: 10.34172/apb.2023.043. Epub 2022 Jan 8. Adv Pharm Bull. 2023. PMID: 37342380 Free PMC article.
-
Gene Therapy in ALS and SMA: Advances, Challenges and Perspectives.Int J Mol Sci. 2023 Jan 6;24(2):1130. doi: 10.3390/ijms24021130. Int J Mol Sci. 2023. PMID: 36674643 Free PMC article. Review.
-
Actin-microtubule cytoskeletal interplay mediated by MRTF-A/SRF signaling promotes dilated cardiomyopathy caused by LMNA mutations.Nat Commun. 2022 Dec 22;13(1):7886. doi: 10.1038/s41467-022-35639-x. Nat Commun. 2022. PMID: 36550158 Free PMC article.
References
-
- Pasinelli P., Brown R.H. Molecular biology of amyotrophic lateral sclerosis: insights from genetics. Nat. Rev. Neurosci. 2006;7:710–723. - PubMed
-
- Bensimon G., Lacomblez L., Meininger V., ALS/Riluzole Study Group A controlled trial of riluzole in amyotrophic lateral sclerosis. N. Engl. J. Med. 1994;330:585–591. - PubMed
-
- Rosen D.R., Siddique T., Patterson D., Figlewicz D.A., Sapp P., Hentati A., Donaldson D., Goto J., O’Regan J.P., Deng H.X. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362:59–62. - PubMed
-
- Scarrott J.M., Herranz-Martín S., Alrafiah A.R., Shaw P.J., Azzouz M. Current developments in gene therapy for amyotrophic lateral sclerosis. Expert Opin. Biol. Ther. 2015;15:935–947. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

