Electron cryo-microscopy structure of the mechanotransduction channel NOMPC
- PMID: 28658211
- PMCID: PMC5669069
- DOI: 10.1038/nature22981
Electron cryo-microscopy structure of the mechanotransduction channel NOMPC
Abstract
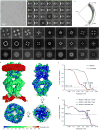
Mechanosensory transduction for senses such as proprioception, touch, balance, acceleration, hearing and pain relies on mechanotransduction channels, which convert mechanical stimuli into electrical signals in specialized sensory cells. How force gates mechanotransduction channels is a central question in the field, for which there are two major models. One is the membrane-tension model: force applied to the membrane generates a change in membrane tension that is sufficient to gate the channel, as in the bacterial MscL channel and certain eukaryotic potassium channels. The other is the tether model: force is transmitted via a tether to gate the channel. The transient receptor potential (TRP) channel NOMPC is important for mechanosensation-related behaviours such as locomotion, touch and sound sensation across different species including Caenorhabditis elegans, Drosophila and zebrafish. NOMPC is the founding member of the TRPN subfamily, and is thought to be gated by tethering of its ankyrin repeat domain to microtubules of the cytoskeleton. Thus, a goal of studying NOMPC is to reveal the underlying mechanism of force-induced gating, which could serve as a paradigm of the tether model. NOMPC fulfils all the criteria that apply to mechanotransduction channels and has 29 ankyrin repeats, the largest number among TRP channels. A key question is how the long ankyrin repeat domain is organized as a tether that can trigger channel gating. Here we present a de novo atomic structure of Drosophila NOMPC determined by single-particle electron cryo-microscopy. Structural analysis suggests that the ankyrin repeat domain of NOMPC resembles a helical spring, suggesting its role of linking mechanical displacement of the cytoskeleton to the opening of the channel. The NOMPC architecture underscores the basis of translating mechanical force into an electrical signal within a cell.
Figures


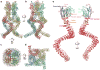



Comment in
-
Structural Biology: A Force-Sensitive Ion Channel Springs to Life.Curr Biol. 2017 Sep 25;27(18):R1017-R1020. doi: 10.1016/j.cub.2017.07.065. Curr Biol. 2017. PMID: 28950085
Similar articles
-
Ankyrin Repeats Convey Force to Gate the NOMPC Mechanotransduction Channel.Cell. 2015 Sep 10;162(6):1391-403. doi: 10.1016/j.cell.2015.08.024. Cell. 2015. PMID: 26359990 Free PMC article.
-
The push-to-open mechanism of the tethered mechanosensitive ion channel NompC.Elife. 2021 Jun 8;10:e58388. doi: 10.7554/eLife.58388. Elife. 2021. PMID: 34101577 Free PMC article.
-
A multiscale model of mechanotransduction by the ankyrin chains of the NOMPC channel.J Gen Physiol. 2019 Mar 4;151(3):316-327. doi: 10.1085/jgp.201812266. Epub 2019 Feb 6. J Gen Physiol. 2019. PMID: 30728217 Free PMC article.
-
Drosophila Mechanosensory Transduction.Trends Neurosci. 2021 Apr;44(4):323-335. doi: 10.1016/j.tins.2020.11.001. Epub 2020 Nov 27. Trends Neurosci. 2021. PMID: 33257000 Review.
-
Forcing open TRP channels: Mechanical gating as a unifying activation mechanism.Biochem Biophys Res Commun. 2015 Apr 24;460(1):22-5. doi: 10.1016/j.bbrc.2015.02.067. Biochem Biophys Res Commun. 2015. PMID: 25998730 Free PMC article. Review.
Cited by
-
Characterization of Lipid-Protein Interactions and Lipid-Mediated Modulation of Membrane Protein Function through Molecular Simulation.Chem Rev. 2019 May 8;119(9):6086-6161. doi: 10.1021/acs.chemrev.8b00608. Epub 2019 Apr 12. Chem Rev. 2019. PMID: 30978005 Free PMC article. Review.
-
Structural insights into the molecular mechanism of mouse TRPA1 activation and inhibition.J Gen Physiol. 2018 May 7;150(5):751-762. doi: 10.1085/jgp.201711876. Epub 2018 Apr 27. J Gen Physiol. 2018. PMID: 29703838 Free PMC article.
-
An asymmetric mechanical code ciphers curvature-dependent proprioceptor activity.Sci Adv. 2021 Sep 17;7(38):eabg4617. doi: 10.1126/sciadv.abg4617. Epub 2021 Sep 17. Sci Adv. 2021. PMID: 34533987 Free PMC article.
-
Cryo-EM structures of the mammalian endo-lysosomal TRPML1 channel elucidate the combined regulation mechanism.Protein Cell. 2017 Nov;8(11):834-847. doi: 10.1007/s13238-017-0476-5. Epub 2017 Sep 21. Protein Cell. 2017. PMID: 28936784 Free PMC article.
-
Two Decades of Evolution of Our Understanding of the Transient Receptor Potential Melastatin 2 (TRPM2) Cation Channel.Life (Basel). 2021 Apr 27;11(5):397. doi: 10.3390/life11050397. Life (Basel). 2021. PMID: 33925466 Free PMC article. Review.
References
-
- Árnadóttir J, Chalfie M. Eukaryotic Mechanosensitive Channels. Annu Rev Biophys. 2010;39:111–137. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

