Glioma cells enhance angiogenesis and inhibit endothelial cell apoptosis through the release of exosomes that contain long non-coding RNA CCAT2
- PMID: 28656228
- PMCID: PMC5562059
- DOI: 10.3892/or.2017.5742
Glioma cells enhance angiogenesis and inhibit endothelial cell apoptosis through the release of exosomes that contain long non-coding RNA CCAT2
Abstract
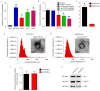
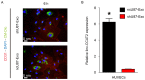
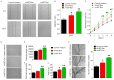
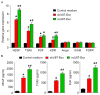
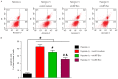
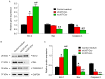
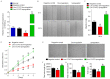
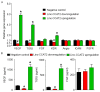
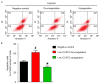
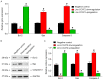
Angiogenesis is a key event in the progression of gliomas. Exosomes, as signaling extracellular organelles, modulate the tumor microenvironment and promote angiogenesis and tumor progression. We previously demonstrated that long intergenic non-coding RNA CCAT2 (linc-CCAT2) was overexpressed in glioma tissues and functioned to promote glioma progression. Therefore, this study aimed to explore an underlying mechanism of glioma cell-affected angiogenesis. First, qRT-PCR was used to determine the expression level of linc-CCAT2 in 4 glioma cell lines and 293T cells, and the results revealed that the U87-MG cells exhibited the highest expression level. Subsequently, the pro-angiogenesis function of exosomes that were derived from negative control shRNA-treated U87-MG cells (ncU87-Exo) and linc-CCAT2 shRNA-treated U87-MG cells (shU87-Exo) was evaluated in vitro and in vivo. We found that ncU87-Exo, which was enriched in linc-CCAT2, could be taken up by HUVECs. ncU87-Exo improved the linc-CCAT2 expression level in HUVECs and more strongly promoted HUVEC migration, proliferation, tubular-like structure formation in vitro and arteriole formation in vivo as well as inhibited HUVEC apoptosis induced by hypoxia. Further mechanistic studies revealed that ncU87-Exo could upregulate VEGFA and TGFβ expression in HUVECs as well as promote Bcl-2 expression and inhibit Bax and caspase-3 expression. Finally, gain-/loss-of-function studies revealed that the overexpression of linc-CCAT2 in HUVECs activated VEGFA and TGFβ, promoted angiogenesis, promoted Bcl-2 expression and inhibited Bax and caspase-3 expression, thus decreasing apoptosis. Downregulation of linc-CCAT2 revealed the opposite effect. Thus, our results revealed a new exosome‑mediated mechanism by which glioma cells could promote angiogenesis through the transfer of linc-CCAT2 by exosomes to endothelial cells. Moreover, we suggest that exosomes and linc-CCAT2 are putative therapeutic targets in glioma.
Figures
Similar articles
-
Glioma cells promote angiogenesis through the release of exosomes containing long non-coding RNA POU3F3.Eur Rev Med Pharmacol Sci. 2017 Mar;21(5):959-972. Eur Rev Med Pharmacol Sci. 2017. PMID: 28338200
-
LncRNA CCAT2 promotes angiogenesis in glioma through activation of VEGFA signalling by sponging miR-424.Mol Cell Biochem. 2020 May;468(1-2):69-82. doi: 10.1007/s11010-020-03712-y. Epub 2020 Apr 1. Mol Cell Biochem. 2020. PMID: 32236863
-
[Long non-coding RNA colon cancer associated transcript-2 from nasopharyngeal carcinoma-derived exosomes promotes angiogenesis].Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2020 Oct 7;55(10):944-951. doi: 10.3760/cma.j.cn115330-20200423-00322. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2020. PMID: 33036509 Chinese.
-
Exosomal noncoding RNAs in Glioma: biological functions and potential clinical applications.Mol Cancer. 2020 Mar 25;19(1):66. doi: 10.1186/s12943-020-01189-3. Mol Cancer. 2020. PMID: 32213181 Free PMC article. Review.
-
CCAT2: A novel oncogenic long non-coding RNA in human cancers.Cell Prolif. 2017 Jun;50(3):e12342. doi: 10.1111/cpr.12342. Epub 2017 Feb 28. Cell Prolif. 2017. PMID: 28244168 Free PMC article. Review.
Cited by
-
Studies on the Regulatory Roles and Related Mechanisms of lncRNAs in the Nervous System.Oxid Med Cell Longev. 2021 Mar 13;2021:6657944. doi: 10.1155/2021/6657944. eCollection 2021. Oxid Med Cell Longev. 2021. PMID: 33791072 Free PMC article. Review.
-
Tumor-derived systems as novel biomedical tools-turning the enemy into an ally.Biomater Res. 2023 Nov 9;27(1):113. doi: 10.1186/s40824-023-00445-z. Biomater Res. 2023. PMID: 37946275 Free PMC article. Review.
-
Long non-coding RNA colon cancer-associated transcript 2 may promote esophageal cancer growth and metastasis by regulating the Wnt signaling pathway.Oncol Lett. 2019 Aug;18(2):1745-1754. doi: 10.3892/ol.2019.10488. Epub 2019 Jun 18. Oncol Lett. 2019. PMID: 31423241 Free PMC article.
-
Can Soluble Immune Checkpoint Molecules on Exosomes Mediate Inflammation?J Neuroimmune Pharmacol. 2022 Dec;17(3-4):381-397. doi: 10.1007/s11481-021-10018-3. Epub 2021 Oct 25. J Neuroimmune Pharmacol. 2022. PMID: 34697721 Free PMC article. Review.
-
The Roles of the Colon Cancer Associated Transcript 2 (CCAT2) Long Non-Coding RNA in Cancer: A Comprehensive Characterization of the Tumorigenic and Molecular Functions.Int J Mol Sci. 2021 Nov 19;22(22):12491. doi: 10.3390/ijms222212491. Int J Mol Sci. 2021. PMID: 34830370 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

