CD33 Splicing Polymorphism Determines Gemtuzumab Ozogamicin Response in De Novo Acute Myeloid Leukemia: Report From Randomized Phase III Children's Oncology Group Trial AAML0531
- PMID: 28644774
- PMCID: PMC5549451
- DOI: 10.1200/JCO.2016.71.2513
CD33 Splicing Polymorphism Determines Gemtuzumab Ozogamicin Response in De Novo Acute Myeloid Leukemia: Report From Randomized Phase III Children's Oncology Group Trial AAML0531
Abstract
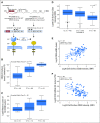
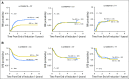
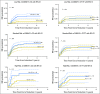
Purpose Gemtuzumab ozogamicin (GO), a CD33-targeted immunoconjugate, is a re-emerging therapy for acute myeloid leukemia (AML). CD33 single nucleotide polymorphism rs12459419 C>T in the splice enhancer region regulates the expression of an alternatively spliced CD33 isoform lacking exon2 (D2-CD33), thus eliminating the CD33 IgV domain, which is the antibody-binding site for GO, as well as diagnostic immunophenotypic panels. We aimed to determine the impact of the genotype of this splicing polymorphism in patients with AML treated with GO-containing chemotherapy. Patients and Methods CD33 splicing single nucleotide polymorphism was evaluated in newly diagnosed patients with AML randomly assigned to receive standard five-course chemotherapy alone (No-GO arm, n = 408) or chemotherapy with the addition of two doses of GO once during induction and once during intensification (GO arm, n = 408) as per the Children's Oncology Group AAML0531 trial. Results The rs12459419 genotype was CC in 415 patients (51%), CT in 316 patients (39%), and TT in 85 patients (10%), with a minor allele frequency of 30%. The T allele was significantly associated with higher levels of D2-CD33 transcript ( P < 1.0E-6) and with lower diagnostic leukemic cell surface CD33 intensity ( P < 1.0E-6). Patients with the CC genotype had significantly lower relapse risk in the GO arm than in the No-GO arm (26% v 49%; P < .001). However, in patients with the CT or TT genotype, exposure to GO did not influence relapse risk (39% v 40%; P = .85). Disease-free survival was higher in patients with the CC genotype in the GO arm than in the No-GO arm (65% v 46%, respectively; P = .004), but this benefit of GO addition was not seen in patients with the CT or TT genotype. Conclusion Our results suggest that patients with the CC genotype for rs12459419 have a substantial response to GO, making this a potential biomarker for the selection of patients with a likelihood of significant response to GO.
Figures
Similar articles
-
Clinical significance of CD33 nonsynonymous single-nucleotide polymorphisms in pediatric patients with acute myeloid leukemia treated with gemtuzumab-ozogamicin-containing chemotherapy.Clin Cancer Res. 2013 Mar 15;19(6):1620-7. doi: 10.1158/1078-0432.CCR-12-3115. Epub 2013 Feb 26. Clin Cancer Res. 2013. PMID: 23444229 Free PMC article.
-
Gemtuzumab ozogamicin in children and adolescents with de novo acute myeloid leukemia improves event-free survival by reducing relapse risk: results from the randomized phase III Children’s Oncology Group trial AAML0531.J Clin Oncol. 2014 Sep 20;32(27):3021-32. doi: 10.1200/JCO.2014.55.3628. J Clin Oncol. 2014. PMID: 25092781 Free PMC article. Clinical Trial.
-
CD33 Expression and Its Association With Gemtuzumab Ozogamicin Response: Results From the Randomized Phase III Children's Oncology Group Trial AAML0531.J Clin Oncol. 2016 Mar 1;34(7):747-55. doi: 10.1200/JCO.2015.62.6846. Epub 2016 Jan 19. J Clin Oncol. 2016. PMID: 26786921 Free PMC article. Clinical Trial.
-
Gemtuzumab ozogamicin in acute myeloid leukemia.Leukemia. 2017 Sep;31(9):1855-1868. doi: 10.1038/leu.2017.187. Epub 2017 Jun 13. Leukemia. 2017. PMID: 28607471 Review.
-
Gemtuzumab ozogamicin for the treatment of acute myeloid leukemia.Expert Rev Clin Pharmacol. 2018 Jun;11(6):549-559. doi: 10.1080/17512433.2018.1478725. Epub 2018 Jun 11. Expert Rev Clin Pharmacol. 2018. PMID: 29787320 Free PMC article. Review.
Cited by
-
The function of alternative splicing in the proteome: rewiring protein interactomes to put old functions into new contexts.Nat Struct Mol Biol. 2023 Dec;30(12):1844-1856. doi: 10.1038/s41594-023-01155-9. Epub 2023 Nov 30. Nat Struct Mol Biol. 2023. PMID: 38036695 Review.
-
MDSC targeting with Gemtuzumab ozogamicin restores T cell immunity and immunotherapy against cancers.EBioMedicine. 2019 Sep;47:235-246. doi: 10.1016/j.ebiom.2019.08.025. Epub 2019 Aug 25. EBioMedicine. 2019. PMID: 31462392 Free PMC article.
-
Acquired Resistance to Antibody-Drug Conjugates.Cancers (Basel). 2019 Mar 20;11(3):394. doi: 10.3390/cancers11030394. Cancers (Basel). 2019. PMID: 30897808 Free PMC article. Review.
-
Drug-regulated CD33-targeted CAR T cells control AML using clinically optimized rapamycin dosing.J Clin Invest. 2024 Mar 19;134(9):e162593. doi: 10.1172/JCI162593. J Clin Invest. 2024. PMID: 38502193 Free PMC article. Clinical Trial.
-
Advances in Acute Myeloid Leukemia: Recently Approved Therapies and Drugs in Development.Cancers (Basel). 2020 Nov 1;12(11):3225. doi: 10.3390/cancers12113225. Cancers (Basel). 2020. PMID: 33139625 Free PMC article. Review.
References
-
- Krupka C, Kufer P, Kischel R, et al. : CD33 target validation and sustained depletion of AML blasts in long-term cultures by the bispecific T-cell-engaging antibody AMG 330. Blood 123:356-365, 2014 - PubMed
-
- Bross PF, Beitz J, Chen G, et al. : Approval summary: Gemtuzumab ozogamicin in relapsed acute myeloid leukemia. Clin Cancer Res 7:1490-1496, 2001 - PubMed
-
- Burnett AK, Hills RK, Hunter AE, et al. : The addition of gemtuzumab ozogamicin to low-dose Ara-C improves remission rate but does not significantly prolong survival in older patients with acute myeloid leukaemia: Results from the LRF AML14 and NCRI AML16 pick-a-winner comparison. Leukemia 27:75-81, 2013 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

