Exosomes: novel regulators of bone remodelling and potential therapeutic agents for orthodontics
- PMID: 28643924
- PMCID: PMC5484069
- DOI: 10.1111/ocr.12165
Exosomes: novel regulators of bone remodelling and potential therapeutic agents for orthodontics
Abstract
Recent studies suggest that exosomes are involved in intercellular communication required for the maintenance of healthy bone. Exosomes are small (30-150 nm in diameter) extracellular vesicles that are formed in multivesicular bodies and are released from cells as the multivesicular bodies fuse with the plasma membrane. Regulatory exosomes have the capacity to exert profound control over target cells. They can stimulate plasma membrane receptors and are also internalized by the target cell delivering proteins, lipids, small molecules and functional RNAs from the cell of origin. We and others have recently reported on regulatory exosomes from osteoclasts and osteoblasts. Key candidate molecules identified in exosome-based regulation of bone remodelling include receptor activator of nuclear factor kappa B (RANK), RANK-ligand (RANKL), ephrinA2, semaphorin 4D, microRNA-146a and microRNA- 214-3p. Exosomes will likely prove to be crucial elements in the communication networks integrating bone cells (osteoclasts, osteoblasts, osteocytes) and linking bone to other tissue. Exosomes collected from bone cells grown in culture may prove useful to augment bone remodelling associated with orthodontic force application or required for the repair of craniofacial bone. Various technologies allow exosomes to be engineered to improve their targeting and efficacy for therapeutic purposes. In summary, exosomes have emerged as important elements of the machinery for intercellular communication between bone cells. They hold great promise as therapeutic targets, biomarkers and therapeutic agents for orthodontists.
Keywords: RANK; extracellular vesicle; microvesicle; osteoblast; osteoclast.
© 2017 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
Figures
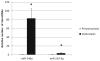
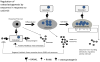

Similar articles
-
RANKL and RANK in extracellular vesicles: surprising new players in bone remodeling.Extracell Vesicles Circ Nucl Acids. 2021;2(1):18-28. doi: 10.20517/evcna.2020.02. Epub 2021 Mar 30. Extracell Vesicles Circ Nucl Acids. 2021. PMID: 33982033 Free PMC article.
-
Characterization of Regulatory Extracellular Vesicles from Osteoclasts.J Dent Res. 2016 Jun;95(6):673-9. doi: 10.1177/0022034516633189. Epub 2016 Feb 23. J Dent Res. 2016. PMID: 26908631 Free PMC article.
-
Zebrafish scales respond differently to in vitro dynamic and static acceleration: analysis of interaction between osteoblasts and osteoclasts.Comp Biochem Physiol A Mol Integr Physiol. 2013 Sep;166(1):74-80. doi: 10.1016/j.cbpa.2013.04.023. Epub 2013 Apr 28. Comp Biochem Physiol A Mol Integr Physiol. 2013. PMID: 23632157
-
The roles of bone-derived exosomes and exosomal microRNAs in regulating bone remodelling.J Cell Mol Med. 2017 May;21(5):1033-1041. doi: 10.1111/jcmm.13039. Epub 2016 Nov 23. J Cell Mol Med. 2017. PMID: 27878944 Free PMC article. Review.
-
Emerging Role of Extracellular Vesicles in Bone Remodeling.J Dent Res. 2018 Jul;97(8):859-868. doi: 10.1177/0022034518764411. Epub 2018 Mar 22. J Dent Res. 2018. PMID: 29566346 Review.
Cited by
-
Role of noncoding RNAs in orthodontic tooth movement: new insights into periodontium remodeling.J Transl Med. 2023 Feb 9;21(1):101. doi: 10.1186/s12967-023-03951-9. J Transl Med. 2023. PMID: 36759852 Free PMC article. Review.
-
Metabolomic signatures distinguish extracellular vesicles from osteoclasts and odontoclasts.Orthod Craniofac Res. 2023 Nov;26(4):632-641. doi: 10.1111/ocr.12658. Epub 2023 Apr 5. Orthod Craniofac Res. 2023. PMID: 36997279 Free PMC article.
-
RANKL and RANK in extracellular vesicles: surprising new players in bone remodeling.Extracell Vesicles Circ Nucl Acids. 2021;2(1):18-28. doi: 10.20517/evcna.2020.02. Epub 2021 Mar 30. Extracell Vesicles Circ Nucl Acids. 2021. PMID: 33982033 Free PMC article.
-
Actin and Actin-Associated Proteins in Extracellular Vesicles Shed by Osteoclasts.Int J Mol Sci. 2019 Dec 25;21(1):158. doi: 10.3390/ijms21010158. Int J Mol Sci. 2019. PMID: 31881680 Free PMC article. Review.
-
Investigating the Differential Circulating microRNA Expression in Adolescent Females with Severe Idiopathic Scoliosis: A Proof-of-Concept Observational Clinical Study.Int J Mol Sci. 2024 Jan 1;25(1):570. doi: 10.3390/ijms25010570. Int J Mol Sci. 2024. PMID: 38203740 Free PMC article.
References
-
- Pan BT, Johnstone RM. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell. 1983;33:967–78. - PubMed
-
- Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

