PD-1 Blockade Promotes Emerging Checkpoint Inhibitors in Enhancing T Cell Responses to Allogeneic Dendritic Cells
- PMID: 28588576
- PMCID: PMC5439058
- DOI: 10.3389/fimmu.2017.00572
PD-1 Blockade Promotes Emerging Checkpoint Inhibitors in Enhancing T Cell Responses to Allogeneic Dendritic Cells
Abstract
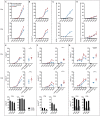
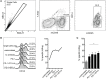
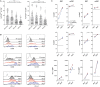
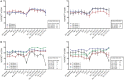
Immune checkpoint inhibitors, which target coinhibitory T cell molecules to promote anticancer immune responses, are on the rise to become a new pillar of cancer therapy. However, current immune checkpoint-based therapies are successful only in a subset of patients and acquired resistances pose additional challenges. Finding new targets and combining checkpoint inhibitors might help to overcome these limitations. In this study, human T cells stimulated with allogeneic dendritic cells (DCs) were used to compare immune checkpoint inhibitors targeting TIM-3, BTLA, LAG-3, CTLA-4, and TIGIT alone or in combination with a PD-1 antibody. We found that PD-1 blockade bears a unique potency to enhance T cell proliferation and cytokine production. Other checkpoint inhibitors failed to significantly augment T cell responses when used alone. However, antibodies to TIM-3, BTLA, LAG-3, and CTLA-4 enhanced T cell proliferation in presence of a PD-1 antibody. Upregulation of coinhibitory T cell receptors upon PD-1 blockade was identified as a potential mechanism for synergistic effects between checkpoint inhibitors. Donor-specific variation in response to immune checkpoint inhibitors was attributed to the T cells rather than DCs. Additionally, we analyzed the regulation of checkpoint molecules and their ligands on T cells and allogeneic DCs in coculture, which suggested a PD-1 blockade-dependent crosstalk between T cells and APC. Our results indicate that several immune checkpoint inhibitors have the capacity to enhance T cell responses when combined with PD-1 blockade. Additional in vitro studies on human T cells will be useful to identify antibody combinations with the potential to augment T cell responses in cancer patients.
Keywords: BTLA; CD160; CTLA-4; LAG-3; PD-1; TIM-3; coinhibitory receptors; immune checkpoint.
Figures


Similar articles
-
Antibodies targeting BTLA or TIM-3 enhance HIV-1 specific T cell responses in combination with PD-1 blockade.Clin Immunol. 2017 Oct;183:167-173. doi: 10.1016/j.clim.2017.09.002. Epub 2017 Sep 4. Clin Immunol. 2017. PMID: 28882621
-
A Comprehensive Analysis of Key Immune Checkpoint Receptors on Tumor-Infiltrating T Cells From Multiple Types of Cancer.Front Oncol. 2019 Oct 25;9:1066. doi: 10.3389/fonc.2019.01066. eCollection 2019. Front Oncol. 2019. PMID: 31709176 Free PMC article.
-
Not All Immune Checkpoints Are Created Equal.Front Immunol. 2018 Aug 31;9:1909. doi: 10.3389/fimmu.2018.01909. eCollection 2018. Front Immunol. 2018. PMID: 30233564 Free PMC article. Review.
-
Profiling the dynamic expression of checkpoint molecules on cytokine-induced killer cells from non-small-cell lung cancer patients.Oncotarget. 2016 Jul 12;7(28):43604-43615. doi: 10.18632/oncotarget.9871. Oncotarget. 2016. PMID: 27283895 Free PMC article.
-
The Next Immune-Checkpoint Inhibitors: PD-1/PD-L1 Blockade in Melanoma.Clin Ther. 2015 Apr 1;37(4):764-82. doi: 10.1016/j.clinthera.2015.02.018. Epub 2015 Mar 29. Clin Ther. 2015. PMID: 25823918 Free PMC article. Review.
Cited by
-
Enhanced antitumor immune response in melanoma tumor model by anti-PD-1 small interference RNA encapsulated in nanoliposomes.Cancer Gene Ther. 2022 Jun;29(6):814-824. doi: 10.1038/s41417-021-00367-9. Epub 2021 Aug 2. Cancer Gene Ther. 2022. PMID: 34341501
-
Tumor microenvironmental influences on dendritic cell and T cell function: A focus on clinically relevant immunologic and metabolic checkpoints.Clin Transl Med. 2020 Jan;10(1):374-411. doi: 10.1002/ctm2.37. Clin Transl Med. 2020. PMID: 32508018 Free PMC article. Review.
-
PD-1/PD-L1 Blockade: Have We Found the Key to Unleash the Antitumor Immune Response?Front Immunol. 2017 Dec 4;8:1597. doi: 10.3389/fimmu.2017.01597. eCollection 2017. Front Immunol. 2017. PMID: 29255458 Free PMC article. Review.
-
Posttransplant complications: molecular mechanisms and therapeutic interventions.MedComm (2020). 2024 Sep 2;5(9):e669. doi: 10.1002/mco2.669. eCollection 2024 Sep. MedComm (2020). 2024. PMID: 39224537 Free PMC article. Review.
-
The Role of Myeloid Cells in Hepatotoxicity Related to Cancer Immunotherapy.Cancers (Basel). 2022 Apr 10;14(8):1913. doi: 10.3390/cancers14081913. Cancers (Basel). 2022. PMID: 35454819 Free PMC article. Review.
References
-
- Coley WB. The treatment of malignant tumors by repeated inoculations of erysipelas. With a report of ten original cases. 1893. Clin Orthop (1991) (262):3–11. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

