Glycomic and glycoproteomic analysis of glycoproteins-a tutorial
- PMID: 28585084
- PMCID: PMC5498624
- DOI: 10.1007/s00216-017-0406-7
Glycomic and glycoproteomic analysis of glycoproteins-a tutorial
Abstract
The structural analysis of glycoproteins is a challenging endeavor and is under steadily increasing demand, but only a very limited number of labs have the expertise required to accomplish this task. This tutorial is aimed at researchers from the fields of molecular biology and biochemistry that have discovered that glycoproteins are important in their biological research and are looking for the tools to elucidate their structure. It provides brief descriptions of the major and most common analytical techniques used in glycomics and glycoproteomics analysis, including explanations of the rationales for individual steps and references to published literature containing the experimental details necessary to carry out the analyses. Glycomics includes the comprehensive study of the structure and function of the glycans expressed in a given cell or organism along with identification of all the genes that encode glycoproteins and glycosyltransferases. Glycoproteomics which is subset of both glycomics and proteomics is the identification and characterization of proteins bearing carbohydrates as posttranslational modification. This tutorial is designed to ease entry into the glycomics and glycoproteomics field for those without prior carbohydrate analysis experience.
Keywords: Glycan analysis; Glycomics; Glycopeptide; Glycoproteomics; Glycosylation site mapping; Mass spectrometry.
Conflict of interest statement
Asif Shajahan, Christian Heiss, and Parastoo Azadi work at the Analytical Services & Training Laboratory at the Complex Carbohydrate Research Center and perform glycomics and glycoproteomics analysis as collaboration or fee-for-service. Mayumi Ishihara declares that she has no conflict of interest.
Figures
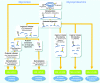
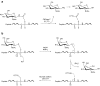
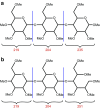
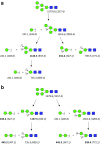
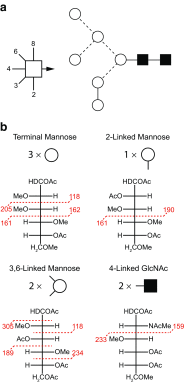

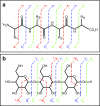
Similar articles
-
Structural separations by ion mobility-MS for glycomics and glycoproteomics.Methods Mol Biol. 2013;951:171-94. doi: 10.1007/978-1-62703-146-2_12. Methods Mol Biol. 2013. PMID: 23296531 Free PMC article.
-
Bioinformatics protocols in glycomics and glycoproteomics.Curr Protoc Protein Sci. 2014 Apr 1;76:2.15.1-2.15.7. doi: 10.1002/0471140864.ps0215s76. Curr Protoc Protein Sci. 2014. PMID: 24692013 Review.
-
Glycomics-informed glycoproteomic analysis of site-specific glycosylation for SARS-CoV-2 spike protein.STAR Protoc. 2020 Dec 15;1(3):100214. doi: 10.1016/j.xpro.2020.100214. eCollection 2020 Dec 18. STAR Protoc. 2020. PMID: 33377107 Free PMC article.
-
Evaluation of a combined glycomics and glycoproteomics approach for studying the major glycoproteins present in biofluids: Application to cerebrospinal fluid.Rapid Commun Mass Spectrom. 2015 Mar 30;29(6):461-73. doi: 10.1002/rcm.7125. Rapid Commun Mass Spectrom. 2015. PMID: 26160412
-
Recent advances in mass spectrometry-based glycoproteomics.Adv Protein Chem Struct Biol. 2014;95:71-123. doi: 10.1016/B978-0-12-800453-1.00003-8. Adv Protein Chem Struct Biol. 2014. PMID: 24985770 Review.
Cited by
-
Functional genomics identifies N-acetyllactosamine extension of complex N-glycans as a mechanism to evade lysis by natural killer cells.Cell Rep. 2024 Apr 23;43(4):114105. doi: 10.1016/j.celrep.2024.114105. Epub 2024 Apr 14. Cell Rep. 2024. PMID: 38619967 Free PMC article.
-
Tool for Rapid Analysis of Glycopeptide by Permethylation via One-Pot Site Mapping and Glycan Analysis.Anal Chem. 2017 Oct 17;89(20):10734-10743. doi: 10.1021/acs.analchem.7b01730. Epub 2017 Oct 2. Anal Chem. 2017. PMID: 28921966 Free PMC article.
-
The glycosyltransferase ST3GAL4 drives immune evasion in acute myeloid leukemia by synthesizing ligands for the glyco-immune checkpoint receptor Siglec-9.Leukemia. 2024 Nov 17. doi: 10.1038/s41375-024-02454-w. Online ahead of print. Leukemia. 2024. PMID: 39551873
-
Reduction in N-Acetylglucosaminyltransferase-I Activity Decreases Survivability and Delays Development of Zebrafish.Curr Issues Mol Biol. 2023 Nov 15;45(11):9165-9180. doi: 10.3390/cimb45110575. Curr Issues Mol Biol. 2023. PMID: 37998752 Free PMC article.
-
Involvement of the Streptococcus mutans PgfE and GalE 4-epimerases in protein glycosylation, carbon metabolism, and cell division.Glycobiology. 2023 Apr 19;33(3):245-259. doi: 10.1093/glycob/cwad004. Glycobiology. 2023. PMID: 36637425 Free PMC article.
References
-
- Guerrini M, Raman R, Venkataraman G, Torri G, Sasisekharan R, Casu B. A novel computational approach to integrate NMR spectroscopy and capillary electrophoresis for structure assignment of heparin and heparan sulfate oligosaccharides. Glycobiology. 2002;12:713–719. doi: 10.1093/glycob/cwf084. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

