Arf6 and Rab22 mediate T cell conjugate formation by regulating clathrin-independent endosomal membrane trafficking
- PMID: 28584192
- PMCID: PMC5536918
- DOI: 10.1242/jcs.200477
Arf6 and Rab22 mediate T cell conjugate formation by regulating clathrin-independent endosomal membrane trafficking
Abstract
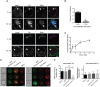
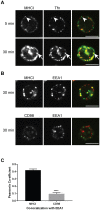
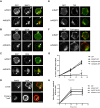
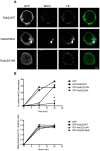
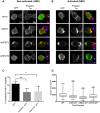
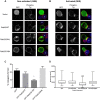
Endosomal trafficking can influence the composition of the plasma membrane and the ability of cells to polarize their membranes. Here, we examined whether trafficking through clathrin-independent endocytosis (CIE) affects the ability of T cells to form a cell-cell conjugate with antigen-presenting cells (APCs). We show that CIE occurs in both the Jurkat T cell line and primary human T cells. In Jurkat cells, the activities of two guanine nucleotide binding proteins, Arf6 and Rab22 (also known as Rab22a), influence CIE and conjugate formation. Expression of the constitutively active form of Arf6, Arf6Q67L, inhibits CIE and conjugate formation, and results in the accumulation of vacuoles containing lymphocyte function-associated antigen 1 (LFA-1) and CD4, molecules important for T cell interaction with the APC. Moreover, expression of the GTP-binding defective mutant of Rab22, Rab22S19N, inhibits CIE and conjugate formation, suggesting that Rab22 function is required for these activities. Furthermore, Jurkat cells expressing Rab22S19N were impaired in spreading onto coverslips coated with T cell receptor-activating antibodies. These observations support a role for CIE, Arf6 and Rab22 in conjugate formation between T cells and APCs.
Keywords: Arf6; Clathrin-independent endocytosis; Immunological synapse; Rab22; Rab22a; T cell.
© 2017. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures
Similar articles
-
Hook1, microtubules, and Rab22: mediators of selective sorting of clathrin-independent endocytic cargo proteins on endosomes.Bioarchitecture. 2013 Sep-Dec;3(5):141-6. doi: 10.4161/bioa.26638. Epub 2013 Sep 1. Bioarchitecture. 2013. PMID: 24284901 Free PMC article.
-
Sorting of Clathrin-Independent Cargo Proteins Depends on Rab35 Delivered by Clathrin-Mediated Endocytosis.Traffic. 2015 Sep;16(9):994-1009. doi: 10.1111/tra.12302. Epub 2015 Jun 4. Traffic. 2015. PMID: 25988331 Free PMC article.
-
Rab and Arf G proteins in endosomal trafficking.Methods Cell Biol. 2015;130:127-38. doi: 10.1016/bs.mcb.2015.04.004. Epub 2015 Jun 11. Methods Cell Biol. 2015. PMID: 26360032 Free PMC article.
-
Clathrin-independent endocytosis: a unique platform for cell signaling and PM remodeling.Cell Signal. 2009 Jan;21(1):1-6. doi: 10.1016/j.cellsig.2008.06.020. Epub 2008 Jul 3. Cell Signal. 2009. PMID: 18647649 Free PMC article. Review.
-
[Clathrin-independent endocytosis - role in disease processes and pharmaceutical aspects].Postepy Hig Med Dosw (Online). 2015 Jul 7;69:763-76. doi: 10.5604/17322693.1160614. Postepy Hig Med Dosw (Online). 2015. PMID: 26206992 Review. Polish.
Cited by
-
Endocytic Recycling of MHC Class I Molecules in Non-professional Antigen Presenting and Dendritic Cells.Front Immunol. 2019 Jan 7;9:3098. doi: 10.3389/fimmu.2018.03098. eCollection 2018. Front Immunol. 2019. PMID: 30666258 Free PMC article. Review.
-
CRACR2a is a calcium-activated dynein adaptor protein that regulates endocytic traffic.J Cell Biol. 2019 May 6;218(5):1619-1633. doi: 10.1083/jcb.201806097. Epub 2019 Feb 27. J Cell Biol. 2019. PMID: 30814157 Free PMC article.
-
Membrane protein trafficking in the anti-tumor immune response: work of endosomal-lysosomal system.Cancer Cell Int. 2022 Dec 17;22(1):413. doi: 10.1186/s12935-022-02805-6. Cancer Cell Int. 2022. PMID: 36528587 Free PMC article. Review.
-
The role of MHC class I recycling and Arf6 in cross-presentation by murine dendritic cells.Life Sci Alliance. 2019 Nov 18;2(6):e201900464. doi: 10.26508/lsa.201900464. Print 2019 Dec. Life Sci Alliance. 2019. PMID: 31740564 Free PMC article.
-
Machine learning/molecular dynamic protein structure prediction approach to investigate the protein conformational ensemble.Sci Rep. 2022 Jun 15;12(1):10018. doi: 10.1038/s41598-022-13714-z. Sci Rep. 2022. PMID: 35705565 Free PMC article.
References
-
- Bouchet J., del Rio-Iñiguez I., Lasserre R., Agüera-Gonzalez S., Cuche C., Danckaert A., McCaffrey M. W., Di Bartolo V. and Alcover A. (2016). Rac1-Rab11-FIP3 regulatory hub coordinates vesicle traffic with actin remodeling and T-cell activation. EMBO J. 35, 1160-1174. 10.15252/embj.201593274 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

