A phase I study of single-agent perifosine for recurrent or refractory pediatric CNS and solid tumors
- PMID: 28582410
- PMCID: PMC5459446
- DOI: 10.1371/journal.pone.0178593
A phase I study of single-agent perifosine for recurrent or refractory pediatric CNS and solid tumors
Abstract
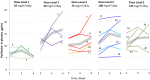
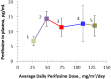
The PI3K/Akt/mTOR signaling pathway is aberrantly activated in various pediatric tumors. We conducted a phase I study of the Akt inhibitor perifosine in patients with recurrent/refractory pediatric CNS and solid tumors. This was a standard 3+3 open-label dose-escalation study to assess pharmacokinetics, describe toxicities, and identify the MTD for single-agent perifosine. Five dose levels were investigated, ranging from 25 to 125 mg/m2/day for 28 days per cycle. Twenty-three patients (median age 10 years, range 4-18 years) with CNS tumors (DIPG [n = 3], high-grade glioma [n = 5], medulloblastoma [n = 2], ependymoma [n = 3]), neuroblastoma (n = 8), Wilms tumor (n = 1), and Ewing sarcoma (n = 1) were treated. Only one DLT occurred (grade 4 hyperuricemia at dose level 4). The most common grade 3 or 4 toxicity at least possibly related to perifosine was neutropenia (8.7%), with the remaining grade 3 or 4 toxicities (fatigue, hyperglycemia, fever, hyperuricemia, and catheter-related infection) occurring in one patient each. Pharmacokinetics was dose-saturable at doses above 50 mg/m2/day with significant inter-patient variability, consistent with findings reported in adult studies. One patient with DIPG (dose level 5) and 4 of 5 patients with high-grade glioma (dose levels 2 and 3) experienced stable disease for two months. Five subjects with neuroblastoma (dose levels 1 through 4) achieved stable disease which was prolonged (≥11 months) in three. No objective responses were noted. In conclusion, the use of perifosine was safe and feasible in patients with recurrent/refractory pediatric CNS and solid tumors. An MTD was not defined by the 5 dose levels investigated. Our RP2D is 50 mg/m2/day.
Conflict of interest statement
Figures
Similar articles
-
A phase I study of perifosine with temsirolimus for recurrent pediatric solid tumors.Pediatr Blood Cancer. 2017 Jul;64(7). doi: 10.1002/pbc.26409. Epub 2016 Dec 30. Pediatr Blood Cancer. 2017. PMID: 28035748 Clinical Trial.
-
A phase 1 study of entinostat in children and adolescents with recurrent or refractory solid tumors, including CNS tumors: Trial ADVL1513, Pediatric Early Phase-Clinical Trial Network (PEP-CTN).Pediatr Blood Cancer. 2021 Apr;68(4):e28892. doi: 10.1002/pbc.28892. Epub 2021 Jan 12. Pediatr Blood Cancer. 2021. PMID: 33438318 Free PMC article. Clinical Trial.
-
Phase I study of bevacizumab plus irinotecan in pediatric patients with recurrent/refractory solid tumors.Jpn J Clin Oncol. 2013 Nov;43(11):1073-9. doi: 10.1093/jjco/hyt124. Epub 2013 Sep 3. Jpn J Clin Oncol. 2013. PMID: 24002900 Clinical Trial.
-
Radiotherapy for pediatric central nervous system tumors: a regional cancer centre experience.J Neurooncol. 2004 Jul;68(3):285-94. doi: 10.1023/b:neon.0000033386.38403.3b. J Neurooncol. 2004. PMID: 15332333 Review.
-
Adjuvant therapies of postsurgical minimal residual disease.Recent Results Cancer Res. 1980;74:8-25. doi: 10.1007/978-3-642-81488-4_2. Recent Results Cancer Res. 1980. PMID: 6255519 Review.
Cited by
-
Regorafenib is effective against neuroblastoma in vitro and in vivo and inhibits the RAS/MAPK, PI3K/Akt/mTOR and Fos/Jun pathways.Br J Cancer. 2020 Aug;123(4):568-579. doi: 10.1038/s41416-020-0905-8. Epub 2020 May 27. Br J Cancer. 2020. PMID: 32457362 Free PMC article.
-
Characterization of prevalent tyrosine kinase inhibitors and their challenges in glioblastoma treatment.Front Chem. 2024 Jan 8;11:1325214. doi: 10.3389/fchem.2023.1325214. eCollection 2023. Front Chem. 2024. PMID: 38264122 Free PMC article. Review.
-
In Vitro Study of Cytotoxic Mechanisms of Alkylphospholipids and Alkyltriazoles in Acute Lymphoblastic Leukemia Models.Molecules. 2022 Dec 6;27(23):8633. doi: 10.3390/molecules27238633. Molecules. 2022. PMID: 36500726 Free PMC article.
-
Synthetic Heterocyclic Derivatives as Kinase Inhibitors Tested for the Treatment of Neuroblastoma.Molecules. 2021 Nov 23;26(23):7069. doi: 10.3390/molecules26237069. Molecules. 2021. PMID: 34885651 Free PMC article. Review.
-
Mitochondrial Targeting Involving Cholesterol-Rich Lipid Rafts in the Mechanism of Action of the Antitumor Ether Lipid and Alkylphospholipid Analog Edelfosine.Pharmaceutics. 2021 May 20;13(5):763. doi: 10.3390/pharmaceutics13050763. Pharmaceutics. 2021. PMID: 34065546 Free PMC article. Review.
References
-
- Luo J, Manning BD, Cantley LC. Targeting the PI3K-Akt pathway in human cancer: rationale and promise. Cancer Cell. 2003;4:257–62. - PubMed
-
- Pollack IF, Hamilton RL, Burger PC, Brat DJ, Rosenblum MK, Murdoch GH, et al. Akt activation is a common event in pediatric malignant gliomas and a potential adverse prognostic marker: a report from the Children's Oncology Group. J Neurooncol. 2010;99:155–63. 10.1007/s11060-010-0297-3 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

