The non-canonical NF-κB pathway in immunity and inflammation
- PMID: 28580957
- PMCID: PMC5753586
- DOI: 10.1038/nri.2017.52
The non-canonical NF-κB pathway in immunity and inflammation
Abstract
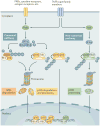
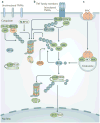
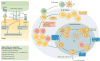
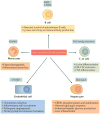
The nuclear factor-κB (NF-κB) family of transcription factors is activated by canonical and non-canonical signalling pathways, which differ in both signalling components and biological functions. Recent studies have revealed important roles for the non-canonical NF-κB pathway in regulating different aspects of immune functions. Defects in non-canonical NF-κB signalling are associated with severe immune deficiencies, whereas dysregulated activation of this pathway contributes to the pathogenesis of various autoimmune and inflammatory diseases. Here we review the signalling mechanisms and the biological function of the non-canonical NF-κB pathway. We also discuss recent progress in elucidating the molecular mechanisms regulating non-canonical NF-κB pathway activation, which may provide new opportunities for therapeutic strategies.
Figures

Similar articles
-
Non-canonical NF-κB signaling activation and regulation: principles and perspectives.Immunol Rev. 2011 Nov;244(1):44-54. doi: 10.1111/j.1600-065X.2011.01059.x. Immunol Rev. 2011. PMID: 22017430 Review.
-
Manipulation of Non-canonical NF-κB Signaling by Non-oncogenic Viruses.Arch Immunol Ther Exp (Warsz). 2019 Feb;67(1):41-48. doi: 10.1007/s00005-018-0522-x. Epub 2018 Sep 8. Arch Immunol Ther Exp (Warsz). 2019. PMID: 30196473 Free PMC article. Review.
-
Linear ubiquitination-mediated NF-κB regulation and its related disorders.J Biochem. 2013 Oct;154(4):313-23. doi: 10.1093/jb/mvt079. Epub 2013 Aug 21. J Biochem. 2013. PMID: 23969028 Review.
-
Non-canonical NF-κB signaling in rheumatoid arthritis: Dr Jekyll and Mr Hyde?Arthritis Res Ther. 2015 Jan 28;17(1):15. doi: 10.1186/s13075-015-0527-3. Arthritis Res Ther. 2015. PMID: 25774937 Free PMC article. Review.
-
Targeting NF-κB pathway for the therapy of diseases: mechanism and clinical study.Signal Transduct Target Ther. 2020 Sep 21;5(1):209. doi: 10.1038/s41392-020-00312-6. Signal Transduct Target Ther. 2020. PMID: 32958760 Free PMC article. Review.
Cited by
-
Regulation Progression on Ellagic Acid Improving Poultry Production Performance by Regulating Redox Homeostasis, Inflammatory Response, and Cell Apoptosis.Animals (Basel). 2024 Oct 17;14(20):3009. doi: 10.3390/ani14203009. Animals (Basel). 2024. PMID: 39457938 Free PMC article. Review.
-
The potential of functionalized dressing releasing flavonoids facilitates scar-free healing.Front Med (Lausanne). 2022 Oct 3;9:978120. doi: 10.3389/fmed.2022.978120. eCollection 2022. Front Med (Lausanne). 2022. PMID: 36262272 Free PMC article. Review.
-
Expression of p52, a non-canonical NF-kappaB transcription factor, is associated with poor ovarian cancer prognosis.Biomark Res. 2020 Sep 15;8:45. doi: 10.1186/s40364-020-00227-y. eCollection 2020. Biomark Res. 2020. PMID: 32974032 Free PMC article.
-
Transcription factor-mediated signaling pathways' contribution to the pathology of acute lung injury and acute respiratory distress syndrome.Am J Transl Res. 2020 Sep 15;12(9):5608-5618. eCollection 2020. Am J Transl Res. 2020. PMID: 33042442 Free PMC article.
-
Inherited human RelB deficiency impairs innate and adaptive immunity to infection.Proc Natl Acad Sci U S A. 2024 Sep 10;121(37):e2321794121. doi: 10.1073/pnas.2321794121. Epub 2024 Sep 4. Proc Natl Acad Sci U S A. 2024. PMID: 39231201 Free PMC article.
References
-
- Hayden MS, Ghosh S. Shared principles in NF-κB signaling. Cell. 2008;132:344–362. - PubMed
-
- Lin L, DeMartino GN, Greene WC. Cotranslational biogenesis of NF-κB p50 by the 26S proteasome. Cell. 1998;92:819–828. - PubMed
-
- Vallabhapurapu S, Karin M. Regulation and function of NF-κB transcription factors in the immune system. Annu Rev Immunol. 2009;27:693–733. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

