Overcoming the Immunosuppressive Tumor Microenvironment of Hodgkin Lymphoma Using Chimeric Antigen Receptor T Cells
- PMID: 28576927
- PMCID: PMC5628114
- DOI: 10.1158/2159-8290.CD-16-0850
Overcoming the Immunosuppressive Tumor Microenvironment of Hodgkin Lymphoma Using Chimeric Antigen Receptor T Cells
Abstract
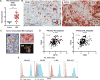
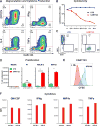
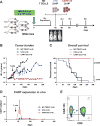
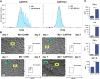
Patients with otherwise treatment-resistant Hodgkin lymphoma could benefit from chimeric antigen receptor T-cell (CART) therapy. However, Hodgkin lymphoma lacks CD19 and contains a highly immunosuppressive tumor microenvironment (TME). We hypothesized that in Hodgkin lymphoma, CART should target both malignant cells and the TME. We demonstrated CD123 on both Hodgkin lymphoma cells and TME, including tumor-associated macrophages (TAM). In vitro, Hodgkin lymphoma cells convert macrophages toward immunosuppressive TAMs that inhibit T-cell proliferation. In contrast, anti-CD123 CART recognized and killed TAMs, thus overcoming immunosuppression. Finally, we showed in immunodeficient mouse models that CART123 eradicated Hodgkin lymphoma and established long-term immune memory. A novel platform that targets malignant cells and the microenvironment may be needed to successfully treat malignancies with an immunosuppressive milieu.Significance: Anti-CD123 chimeric antigen receptor T cells target both the malignant cells and TAMs in Hodgkin lymphoma, thereby eliminating an important immunosuppressive component of the tumor microenvironment. Cancer Discov; 7(10); 1154-67. ©2017 AACR.This article is highlighted in the In This Issue feature, p. 1047.
©2017 American Association for Cancer Research.
Figures


Similar articles
-
Dual CD19 and CD123 targeting prevents antigen-loss relapses after CD19-directed immunotherapies.J Clin Invest. 2016 Oct 3;126(10):3814-3826. doi: 10.1172/JCI87366. Epub 2016 Aug 29. J Clin Invest. 2016. PMID: 27571406 Free PMC article.
-
Single-Cell Transcriptome Analysis Reveals Disease-Defining T-cell Subsets in the Tumor Microenvironment of Classic Hodgkin Lymphoma.Cancer Discov. 2020 Mar;10(3):406-421. doi: 10.1158/2159-8290.CD-19-0680. Epub 2019 Dec 19. Cancer Discov. 2020. PMID: 31857391
-
Targeting Cancer Cells and Tumor Microenvironment in Preclinical and Clinical Models of Hodgkin Lymphoma Using the Dual PI3Kδ/γ Inhibitor RP6530.Clin Cancer Res. 2019 Feb 1;25(3):1098-1112. doi: 10.1158/1078-0432.CCR-18-1133. Epub 2018 Oct 23. Clin Cancer Res. 2019. PMID: 30352904
-
The circuitry of the tumor microenvironment in adult and pediatric Hodgkin lymphoma: cellular composition, cytokine profile, EBV, and exosomes.Cancer Rep (Hoboken). 2021 Apr;4(2):e1311. doi: 10.1002/cnr2.1311. Epub 2020 Oct 26. Cancer Rep (Hoboken). 2021. PMID: 33103852 Free PMC article. Review.
-
Building upon the success of CART19: chimeric antigen receptor T cells for hematologic malignancies.Leuk Lymphoma. 2018 Sep;59(9):2040-2055. doi: 10.1080/10428194.2017.1403024. Epub 2017 Nov 22. Leuk Lymphoma. 2018. PMID: 29165008 Free PMC article. Review.
Cited by
-
Complex interplay between tumor microenvironment and cancer therapy.Front Med. 2018 Aug;12(4):426-439. doi: 10.1007/s11684-018-0663-7. Epub 2018 Aug 10. Front Med. 2018. PMID: 30097962 Review.
-
Role and Mechanisms of Tumor-Associated Macrophages in Hematological Malignancies.Front Oncol. 2022 Jul 7;12:933666. doi: 10.3389/fonc.2022.933666. eCollection 2022. Front Oncol. 2022. PMID: 35875135 Free PMC article. Review.
-
Quantitative PET-based biomarkers in lymphoma: getting ready for primetime.Nat Rev Clin Oncol. 2023 Sep;20(9):640-657. doi: 10.1038/s41571-023-00799-2. Epub 2023 Jul 17. Nat Rev Clin Oncol. 2023. PMID: 37460635 Review.
-
CAR-T cell-mediated depletion of immunosuppressive tumor-associated macrophages promotes endogenous antitumor immunity and augments adoptive immunotherapy.Nat Commun. 2021 Feb 9;12(1):877. doi: 10.1038/s41467-021-20893-2. Nat Commun. 2021. PMID: 33563975 Free PMC article.
-
Identification of immune-related cells and genes in the breast invasive carcinoma microenvironment.Aging (Albany NY). 2022 Feb 4;14(3):1374-1388. doi: 10.18632/aging.203879. Epub 2022 Feb 4. Aging (Albany NY). 2022. PMID: 35120331 Free PMC article.
References
-
- Josting A, Franklin J, May M, Koch P, Beykirch MK, Heinz J, et al. New prognostic score based on treatment outcome of patients with relapsed Hodgkin's lymphoma registered in the database of the German Hodgkin's lymphoma study group. J Clin Oncol. 2002;20:221–30. - PubMed
-
- Santoro A, Bonadonna G, Valagussa P, Zucali R, Viviani S, Villani F, et al. Long-term results of combined chemotherapy-radiotherapy approach in Hodgkin's disease: superiority of ABVD plus radiotherapy versus MOPP plus radiotherapy. J Clin Oncol. 1987;5:27–37. - PubMed
-
- Borchmann S, von Tresckow B. Novel agents in classical Hodgkin lymphoma. Leukemia & lymphoma. 2017:1–12. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

