Activation of the EGF Receptor by Ligand Binding and Oncogenic Mutations: The "Rotation Model"
- PMID: 28574446
- PMCID: PMC5492017
- DOI: 10.3390/cells6020013
Activation of the EGF Receptor by Ligand Binding and Oncogenic Mutations: The "Rotation Model"
Abstract
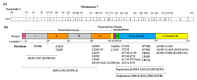
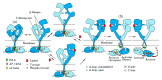
The epidermal growth factor receptor (EGFR) plays vital roles in cellular processes including cell proliferation, survival, motility, and differentiation. The dysregulated activation of the receptor is often implicated in human cancers. EGFR is synthesized as a single-pass transmembrane protein, which consists of an extracellular ligand-binding domain and an intracellular kinase domain separated by a single transmembrane domain. The receptor is activated by a variety of polypeptide ligands such as epidermal growth factor and transforming growth factor α. It has long been thought that EGFR is activated by ligand-induced dimerization of the receptor monomer, which brings intracellular kinase domains into close proximity for trans-autophosphorylation. An increasing number of diverse studies, however, demonstrate that EGFR is present as a pre-formed, yet inactive, dimer prior to ligand binding. Furthermore, recent progress in structural studies has provided insight into conformational changes during the activation of a pre-formed EGFR dimer. Upon ligand binding to the extracellular domain of EGFR, its transmembrane domains rotate or twist parallel to the plane of the cell membrane, resulting in the reorientation of the intracellular kinase domain dimer from a symmetric inactive configuration to an asymmetric active form (the "rotation model"). This model is also able to explain how oncogenic mutations activate the receptor in the absence of the ligand, without assuming that the mutations induce receptor dimerization. In this review, we discuss the mechanisms underlying the ligand-induced activation of the preformed EGFR dimer, as well as how oncogenic mutations constitutively activate the receptor dimer, based on the rotation model.
Keywords: EGFR; cancer; cell-surface receptor; molecular mechanism; phosphorylation; receptor tyrosine kinase; transmembrane signal transduction.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Activation of preformed EGF receptor dimers by ligand-induced rotation of the transmembrane domain.J Mol Biol. 2001 Aug 31;311(5):1011-26. doi: 10.1006/jmbi.2001.4923. J Mol Biol. 2001. PMID: 11531336
-
Allosteric activation of preformed EGF receptor dimers by a single ligand binding event.Front Endocrinol (Lausanne). 2022 Nov 30;13:1042787. doi: 10.3389/fendo.2022.1042787. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36531494 Free PMC article.
-
The ErbB/HER family of protein-tyrosine kinases and cancer.Pharmacol Res. 2014 Jan;79:34-74. doi: 10.1016/j.phrs.2013.11.002. Epub 2013 Nov 20. Pharmacol Res. 2014. PMID: 24269963 Review.
-
Ligand-independent dimer formation of epidermal growth factor receptor (EGFR) is a step separable from ligand-induced EGFR signaling.Mol Biol Cell. 2002 Jul;13(7):2547-57. doi: 10.1091/mbc.01-08-0411. Mol Biol Cell. 2002. PMID: 12134089 Free PMC article.
-
A structural perspective on the regulation of the epidermal growth factor receptor.Annu Rev Biochem. 2015;84:739-64. doi: 10.1146/annurev-biochem-060614-034402. Epub 2015 Jan 26. Annu Rev Biochem. 2015. PMID: 25621509 Free PMC article. Review.
Cited by
-
Modulation of autophagy as a therapeutic strategy for Toxoplasma gondii infection.Front Cell Infect Microbiol. 2022 Aug 24;12:902428. doi: 10.3389/fcimb.2022.902428. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36093185 Free PMC article. Review.
-
A perspective of fluorescence microscopy for cellular structural biology with EGFR as witness.J Microsc. 2023 Jul;291(1):73-91. doi: 10.1111/jmi.13151. Epub 2022 Nov 4. J Microsc. 2023. PMID: 36282005 Free PMC article.
-
Advances in targeting EGFR allosteric site as anti-NSCLC therapy to overcome the drug resistance.Pharmacol Rep. 2020 Aug;72(4):799-813. doi: 10.1007/s43440-020-00131-0. Epub 2020 Jul 14. Pharmacol Rep. 2020. PMID: 32666476 Free PMC article. Review.
-
Expanding the Disorder-Function Paradigm in the C-Terminal Tails of Erbbs.Biomolecules. 2021 Nov 14;11(11):1690. doi: 10.3390/biom11111690. Biomolecules. 2021. PMID: 34827688 Free PMC article. Review.
-
Modulation of Transmembrane Domain Interactions in Neu Receptor Tyrosine Kinase by Membrane Fluidity and Cholesterol.J Membr Biol. 2019 Oct;252(4-5):357-369. doi: 10.1007/s00232-019-00075-4. Epub 2019 Jun 20. J Membr Biol. 2019. PMID: 31222471
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

