miR-183/96 plays a pivotal regulatory role in mouse photoreceptor maturation and maintenance
- PMID: 28559309
- PMCID: PMC5474811
- DOI: 10.1073/pnas.1618757114
miR-183/96 plays a pivotal regulatory role in mouse photoreceptor maturation and maintenance
Abstract
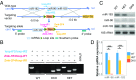
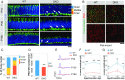
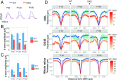
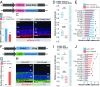
MicroRNAs (miRNAs) are known to be essential for retinal maturation and functionality; however, the role of the most abundant miRNAs, the miR-183/96/182 cluster (miR-183 cluster), in photoreceptor cells remains unclear. Here we demonstrate that ablation of two components of the miR-183 cluster, miR-183 and miR-96, significantly affects photoreceptor maturation and maintenance in mice. Morphologically, early-onset dislocated cone nuclei, shortened outer segments and thinned outer nuclear layers are observed in the miR-183/96 double-knockout (DKO) mice. Abnormal photoreceptor responses, including abolished photopic electroretinography (ERG) responses and compromised scotopic ERG responses, reflect the functional changes in the degenerated retina. We further identify Slc6a6 as the cotarget of miR-183 and miR-96. The expression level of Slc6a6 is significantly higher in the DKO mice than in the wild-type mice. In contrast, Slc6a6 is down-regulated by adeno-associated virus-mediated overexpression of either miR-183 or miR-96 in wild-type mice. Remarkably, both silencing and overexpression of Slc6a6 in the retina are detrimental to the electrophysiological activity of the photoreceptors in response to dim light stimuli. We demonstrate that miR-183/96-mediated fine-tuning of Slc6a6 expression is indispensable for photoreceptor maturation and maintenance, thereby providing insight into the epigenetic regulation of photoreceptors in mice.
Keywords: degeneration; miR-183/96/182 cluster; photoreceptor; regulation; taurine transporter.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Reduced photoreceptor death and improved retinal function during retinal degeneration in mice lacking innate immunity adaptor protein MyD88.Exp Neurol. 2015 May;267:1-12. doi: 10.1016/j.expneurol.2015.02.027. Epub 2015 Feb 25. Exp Neurol. 2015. PMID: 25725353 Free PMC article.
-
Conditional Deletion of Cytoplasmic Dynein Heavy Chain in Postnatal Photoreceptors.Invest Ophthalmol Vis Sci. 2021 Nov 1;62(14):23. doi: 10.1167/iovs.62.14.23. Invest Ophthalmol Vis Sci. 2021. PMID: 34807236 Free PMC article.
-
Ablation of Mature miR-183 Leads to Retinal Dysfunction in Mice.Invest Ophthalmol Vis Sci. 2020 Mar 9;61(3):12. doi: 10.1167/iovs.61.3.12. Invest Ophthalmol Vis Sci. 2020. PMID: 32176259 Free PMC article.
-
The impact of miR-183/182/96 gene regulation on the maturation, survival, and function of photoreceptor cells in the retina.J Comp Neurol. 2020 Jun 15;528(9):1616-1625. doi: 10.1002/cne.24833. Epub 2019 Dec 18. J Comp Neurol. 2020. PMID: 31785157 Review.
-
Conditional gene knockout system in cone photoreceptors.Adv Exp Med Biol. 2006;572:173-8. doi: 10.1007/0-387-32442-9_26. Adv Exp Med Biol. 2006. PMID: 17249572 Review.
Cited by
-
Insight into the role of non-coding RNA in the diagnosis and treatment of retinitis pigmentosa.Noncoding RNA Res. 2023 Oct 29;9(1):44-54. doi: 10.1016/j.ncrna.2023.10.011. eCollection 2024 Mar. Noncoding RNA Res. 2023. PMID: 38075200 Free PMC article. Review.
-
Depletion of miR-96 Delays, But Does Not Arrest, Photoreceptor Development in Mice.Invest Ophthalmol Vis Sci. 2022 Apr 1;63(4):24. doi: 10.1167/iovs.63.4.24. Invest Ophthalmol Vis Sci. 2022. PMID: 35481839 Free PMC article.
-
Sophisticated Gene Regulation for a Complex Physiological System: The Role of Non-coding RNAs in Photoreceptor Cells.Front Cell Dev Biol. 2021 Jan 18;8:629158. doi: 10.3389/fcell.2020.629158. eCollection 2020. Front Cell Dev Biol. 2021. PMID: 33537317 Free PMC article. Review.
-
Regulation of the Glycine Transporter GLYT1 by microRNAs.Neurochem Res. 2022 Jan;47(1):138-147. doi: 10.1007/s11064-021-03228-x. Epub 2021 Jan 23. Neurochem Res. 2022. PMID: 33484385
-
The Circular RNome of Developmental Retina in Mice.Mol Ther Nucleic Acids. 2020 Mar 6;19:339-349. doi: 10.1016/j.omtn.2019.11.016. Epub 2019 Nov 26. Mol Ther Nucleic Acids. 2020. PMID: 31877410 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

