Long-term clinical outcomes following treatment with alpha 1-proteinase inhibitor for COPD associated with alpha-1 antitrypsin deficiency: a look at the evidence
- PMID: 28558837
- PMCID: PMC5450185
- DOI: 10.1186/s12931-017-0574-1
Long-term clinical outcomes following treatment with alpha 1-proteinase inhibitor for COPD associated with alpha-1 antitrypsin deficiency: a look at the evidence
Abstract
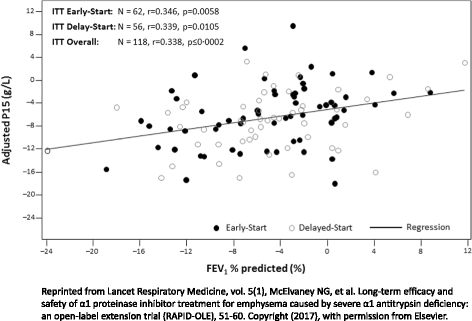
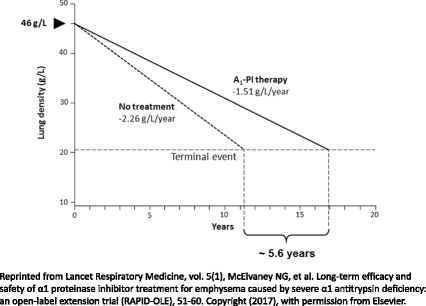
Alpha-1 antitrypsin deficiency (AATD) is a common hereditary disorder caused by mutations in the SERPINA1 gene, which encodes alpha-1 antitrypsin (AAT; also known as alpha 1-proteinase inhibitor, A1-PI). An important function of A1-PI in the lung is to inhibit neutrophil elastase, one of various proteolytic enzymes released by activated neutrophils during inflammation. Absence or deficiency of A1-PI leads to an imbalance between elastase and anti-elastase activity, which results in progressive, irreversible destruction of lung tissue, and ultimately the development of chronic obstructive pulmonary disease with early-onset emphysema. AATD is under-diagnosed, patients can experience long delays before obtaining an accurate diagnosis, and the consequences of delayed diagnosis or misdiagnosis can be severe. Currently, A1-PI therapy is the only available treatment that addresses disease etiology in patients with AATD; however, demonstrating clinical efficacy of A1-PI therapy is challenging. In order to show therapeutic efficacy with traditional endpoints such as forced expiratory volume in one second and mortality, large sample sizes and longer duration trials are required. However, AATD is a rare, slow progressive disease, which can take decades to manifest clinically and recruiting sufficient numbers of patients into prolonged placebo-controlled trials remains a significant obstacle. Despite this, the Randomized, placebo-controlled trial of augmentation therapy in Alpha 1-Proteinase Inhibitor Deficiency (RAPID) and RAPID Extension trial, the largest clinical program completed to date, utilized quantitative chest computed tomography as a sensitive and specific measure of the extent of emphysema. Findings from the RAPID/RAPID Extension program definitively confirmed the benefits of A1-PI therapy in slowing disease progression and provided evidence of a disease-modifying effect of A1-PI therapy in patients with AATD. These findings suggest that the early introduction of treatment in patients with severe emphysema-related AATD may delay the time to death, lung transplantation or crippling respiratory complaints. In addition, there is now limited evidence that A1-PI therapy provides a gain of more than five life-years, supporting previous observations based on registry data. With the clinical efficacy of A1-PI therapy now demonstrated, further studies are required to assess long-term outcomes.
Keywords: Alpha-1 antitrypsin; Alpha-1 antitrypsin deficiency; Chronic obstructive pulmonary disease; Emphysema; Mortality; Outcome; Treatment.
Figures
Similar articles
-
Quantitative disease progression model of α-1 proteinase inhibitor therapy on computed tomography lung density in patients with α-1 antitrypsin deficiency.Br J Clin Pharmacol. 2017 Nov;83(11):2386-2397. doi: 10.1111/bcp.13358. Epub 2017 Aug 11. Br J Clin Pharmacol. 2017. PMID: 28662542 Free PMC article.
-
Treatment of lung disease in alpha-1 antitrypsin deficiency: a systematic review.Int J Chron Obstruct Pulmon Dis. 2017 May 2;12:1295-1308. doi: 10.2147/COPD.S130440. eCollection 2017. Int J Chron Obstruct Pulmon Dis. 2017. PMID: 28496314 Free PMC article. Review.
-
Indications for active case searches and intravenous alpha-1 antitrypsin treatment for patients with alpha-1 antitrypsin deficiency chronic pulmonary obstructive disease: an update.Arch Bronconeumol. 2015 Apr;51(4):185-92. doi: 10.1016/j.arbres.2014.05.008. Epub 2014 Jul 12. Arch Bronconeumol. 2015. PMID: 25027067 English, Spanish.
-
SPARTA clinical trial design: exploring the efficacy and safety of two dose regimens of alpha1-proteinase inhibitor augmentation therapy in alpha1-antitrypsin deficiency.Respir Med. 2015 Apr;109(4):490-9. doi: 10.1016/j.rmed.2015.01.022. Epub 2015 Feb 13. Respir Med. 2015. PMID: 25727857 Clinical Trial.
-
Alpha 1 antitrypsin to treat lung disease in alpha 1 antitrypsin deficiency: recent developments and clinical implications.Int J Chron Obstruct Pulmon Dis. 2018 Jan 31;13:419-432. doi: 10.2147/COPD.S149429. eCollection 2018. Int J Chron Obstruct Pulmon Dis. 2018. PMID: 29430176 Free PMC article. Review.
Cited by
-
Pseudomonas aeruginosa in chronic lung disease: untangling the dysregulated host immune response.Front Immunol. 2024 Jun 28;15:1405376. doi: 10.3389/fimmu.2024.1405376. eCollection 2024. Front Immunol. 2024. PMID: 39015565 Free PMC article. Review.
-
Diagnosis and management of α1-antitrypsin deficiency in Europe: an expert survey.ERJ Open Res. 2019 Mar 11;5(1):00171-2018. doi: 10.1183/23120541.00171-2018. eCollection 2019 Feb. ERJ Open Res. 2019. PMID: 30863774 Free PMC article.
-
Alpha-1 Antitrypsin Substitution for Extrapulmonary Conditions in Alpha-1 Antitrypsin Deficient Patients.Chronic Obstr Pulm Dis. 2018 Sep 19;5(4):267-276. doi: 10.15326/jcopdf.5.4.2017.0161. Chronic Obstr Pulm Dis. 2018. PMID: 30723784 Free PMC article.
-
S-Nitrosylation of α1-Antitrypsin Triggers Macrophages Toward Inflammatory Phenotype and Enhances Intra-Cellular Bacteria Elimination.Front Immunol. 2019 Apr 2;10:590. doi: 10.3389/fimmu.2019.00590. eCollection 2019. Front Immunol. 2019. PMID: 31001247 Free PMC article.
-
Alpha-1 Antitrypsin Deficiency and Accelerated Aging: A New Model for an Old Disease?Drugs Aging. 2019 Sep;36(9):823-840. doi: 10.1007/s40266-019-00684-7. Drugs Aging. 2019. PMID: 31179525 Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

