Multilevel genomics of colorectal cancers with microsatellite instability-clinical impact of JAK1 mutations and consensus molecular subtype 1
- PMID: 28539123
- PMCID: PMC5442873
- DOI: 10.1186/s13073-017-0434-0
Multilevel genomics of colorectal cancers with microsatellite instability-clinical impact of JAK1 mutations and consensus molecular subtype 1
Abstract
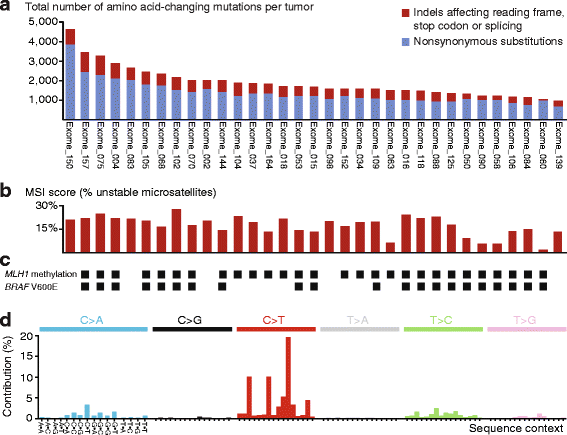
Background: Approximately 15% of primary colorectal cancers have DNA mismatch repair deficiency, causing a complex genome with thousands of small mutations-the microsatellite instability (MSI) phenotype. We investigated molecular heterogeneity and tumor immunogenicity in relation to clinical endpoints within this distinct subtype of colorectal cancers.
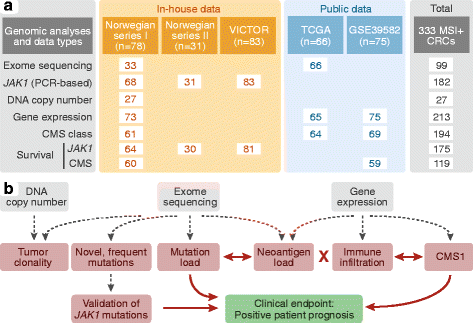
Methods: A total of 333 primary MSI+ colorectal tumors from multiple cohorts were analyzed by multilevel genomics and computational modeling-including mutation profiling, clonality modeling, and neoantigen prediction in a subset of the tumors, as well as gene expression profiling for consensus molecular subtypes (CMS) and immune cell infiltration.
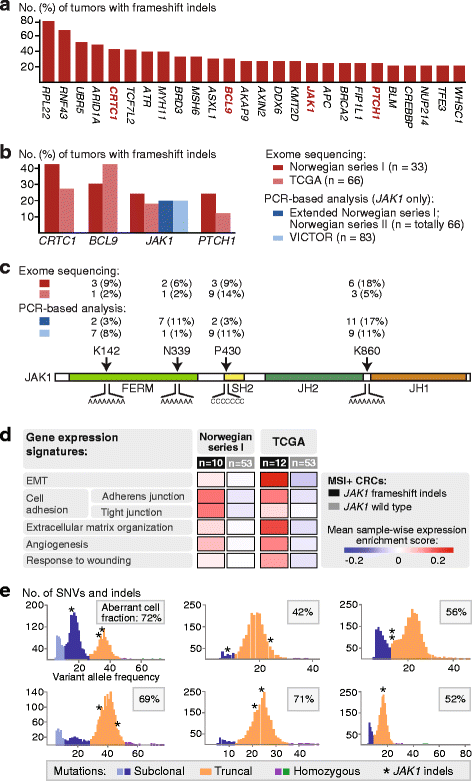
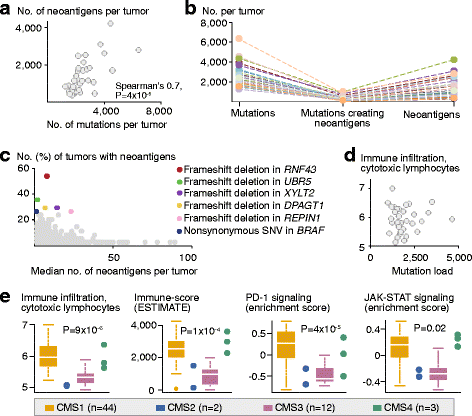
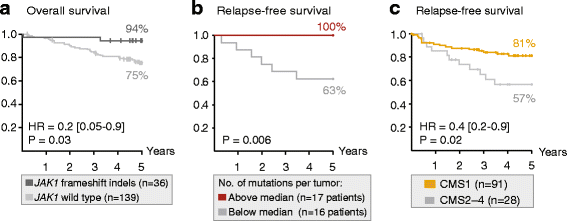
Results: Novel, frequent frameshift mutations in four cancer-critical genes were identified by deep exome sequencing, including in CRTC1, BCL9, JAK1, and PTCH1. JAK1 loss-of-function mutations were validated with an overall frequency of 20% in Norwegian and British patients, and mutated tumors had up-regulation of transcriptional signatures associated with resistance to anti-PD-1 treatment. Clonality analyses revealed a high level of intra-tumor heterogeneity; however, this was not associated with disease progression. Among the MSI+ tumors, the total mutation load correlated with the number of predicted neoantigens (P = 4 × 10-5), but not with immune cell infiltration-this was dependent on the CMS class; MSI+ tumors in CMS1 were highly immunogenic compared to MSI+ tumors in CMS2-4. Both JAK1 mutations and CMS1 were favorable prognostic factors (hazard ratios 0.2 [0.05-0.9] and 0.4 [0.2-0.9], respectively, P = 0.03 and 0.02).
Conclusions: Multilevel genomic analyses of MSI+ colorectal cancer revealed molecular heterogeneity with clinical relevance, including tumor immunogenicity and a favorable patient outcome associated with JAK1 mutations and the transcriptomic subgroup CMS1, emphasizing the potential for prognostic stratification of this clinically important subtype. See related research highlight by Samstein and Chan 10.1186/s13073-017-0438-9.
Keywords: Colorectal cancer; Consensus molecular subtypes; Immunogenicity; Immunotherapy resistance; JAK1; Microsatellite instability; Mutation; Neoantigen; Prognosis.
Figures
Comment in
-
Dissecting microsatellite instability in colorectal cancer: one size does not fit all.Genome Med. 2017 May 24;9(1):45. doi: 10.1186/s13073-017-0438-9. Genome Med. 2017. PMID: 28539127 Free PMC article.
Similar articles
-
CMS-dependent prognostic impact of KRAS and BRAFV600E mutations in primary colorectal cancer.Ann Oncol. 2018 May 1;29(5):1227-1234. doi: 10.1093/annonc/mdy085. Ann Oncol. 2018. PMID: 29518181 Free PMC article.
-
Genomic and transcriptomic characterization of heterogeneous immune subgroups of microsatellite instability-high colorectal cancers.J Immunother Cancer. 2021 Dec;9(12):e003414. doi: 10.1136/jitc-2021-003414. J Immunother Cancer. 2021. PMID: 34903553 Free PMC article.
-
B2M and JAK1/2-mutated MSI-H Colorectal Carcinomas Can Benefit From Anti-PD-1 Therapy.J Immunother. 2022 May 1;45(4):187-193. doi: 10.1097/CJI.0000000000000417. J Immunother. 2022. PMID: 35343934 Free PMC article.
-
The Evolving Role of Consensus Molecular Subtypes: a Step Beyond Inpatient Selection for Treatment of Colorectal Cancer.Curr Treat Options Oncol. 2021 Nov 6;22(12):113. doi: 10.1007/s11864-021-00913-5. Curr Treat Options Oncol. 2021. PMID: 34741675 Review.
-
Molecular pathological classification of colorectal cancer.Virchows Arch. 2016 Aug;469(2):125-34. doi: 10.1007/s00428-016-1956-3. Epub 2016 Jun 20. Virchows Arch. 2016. PMID: 27325016 Free PMC article. Review.
Cited by
-
Mutational signatures impact the evolution of anti-EGFR antibody resistance in colorectal cancer.Nat Ecol Evol. 2021 Jul;5(7):1024-1032. doi: 10.1038/s41559-021-01470-8. Epub 2021 May 20. Nat Ecol Evol. 2021. PMID: 34017094 Free PMC article.
-
Complex pattern of immune evasion in MSI colorectal cancer.Oncoimmunology. 2018 Mar 26;7(7):e1445453. doi: 10.1080/2162402X.2018.1445453. eCollection 2018. Oncoimmunology. 2018. PMID: 29900056 Free PMC article.
-
CMS-dependent prognostic impact of KRAS and BRAFV600E mutations in primary colorectal cancer.Ann Oncol. 2018 May 1;29(5):1227-1234. doi: 10.1093/annonc/mdy085. Ann Oncol. 2018. PMID: 29518181 Free PMC article.
-
Genomic instability as a driver and suppressor of anti-tumor immunity.Front Immunol. 2024 Oct 11;15:1462496. doi: 10.3389/fimmu.2024.1462496. eCollection 2024. Front Immunol. 2024. PMID: 39544936 Free PMC article. Review.
-
Molecular correlates of sensitivity to PARP inhibition beyond homologous recombination deficiency in pre-clinical models of colorectal cancer point to wild-type TP53 activity.EBioMedicine. 2020 Sep;59:102923. doi: 10.1016/j.ebiom.2020.102923. Epub 2020 Aug 13. EBioMedicine. 2020. PMID: 32799124 Free PMC article.
References
-
- Kane MF, Loda M, Gaida GM, Lipman J, Mishra R, Goldman H, et al. Methylation of the hMLH1 promoter correlates with lack of expression of hMLH1 in sporadic colon tumors and mismatch repair-defective human tumor cell lines. Cancer Res. 1997;57:808–11. - PubMed
-
- Cunningham JM, Christensen ER, Tester DJ, Kim CY, Roche PC, Burgart LJ, et al. Hypermethylation of the hMLH1 promoter in colon cancer with microsatellite instability. Cancer Res. 1998;58:3455–60. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

