Hepatocyte polyploidization and its association with pathophysiological processes
- PMID: 28518148
- PMCID: PMC5520697
- DOI: 10.1038/cddis.2017.167
Hepatocyte polyploidization and its association with pathophysiological processes
Abstract
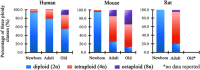
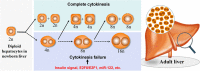
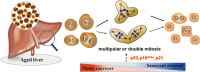
A characteristic cellular feature of the mammalian liver is the progressive polyploidization of the hepatocytes, where individual cells acquire more than two sets of chromosomes. Polyploidization results from cytokinesis failure that takes place progressively during the course of postnatal development. The proportion of polyploidy also increases with the aging process or with cellular stress such as surgical resection, toxic stimulation, metabolic overload, or oxidative damage, to involve as much as 90% of the hepatocytes in mice and 40% in humans. Hepatocyte polyploidization is generally considered an indicator of terminal differentiation and cellular senescence, and related to the dysfunction of insulin and p53/p21 signaling pathways. Interestingly, the high prevalence of hepatocyte polyploidization in the aged mouse liver can be reversed when the senescent hepatocytes are serially transplanted into young mouse livers. Here we review the current knowledge on the mechanism of hepatocytes polyploidization during postnatal growth, aging, and liver diseases. The biologic significance of polyploidization in senescent reversal, within the context of new ways to think of liver aging and liver diseases is considered.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Polyploidy in liver development, homeostasis and disease.Nat Rev Gastroenterol Hepatol. 2020 Jul;17(7):391-405. doi: 10.1038/s41575-020-0284-x. Epub 2020 Apr 2. Nat Rev Gastroenterol Hepatol. 2020. PMID: 32242122 Review.
-
MicroRNA-122 regulates polyploidization in the murine liver.Hepatology. 2016 Aug;64(2):599-615. doi: 10.1002/hep.28573. Epub 2016 May 12. Hepatology. 2016. PMID: 27016325 Free PMC article.
-
Polyploidization of liver cells.Adv Exp Med Biol. 2010;676:123-35. doi: 10.1007/978-1-4419-6199-0_8. Adv Exp Med Biol. 2010. PMID: 20687473 Review.
-
Polyploidization in liver tissue.Am J Pathol. 2014 Feb;184(2):322-31. doi: 10.1016/j.ajpath.2013.06.035. Epub 2013 Oct 17. Am J Pathol. 2014. PMID: 24140012 Review.
-
[The kinetics of the cell population of human liver parenchyma at different periods of life].Tsitologiia. 1991;33(8):96-109. Tsitologiia. 1991. PMID: 1821502 Russian.
Cited by
-
Assessment of Hepatocyte Ploidy by Flow Cytometry.Methods Mol Biol. 2022;2544:171-181. doi: 10.1007/978-1-0716-2557-6_12. Methods Mol Biol. 2022. PMID: 36125718
-
Functional Network of the Long Non-coding RNA Growth Arrest-Specific Transcript 5 and Its Interacting Proteins in Senescence.Front Genet. 2021 Mar 10;12:615340. doi: 10.3389/fgene.2021.615340. eCollection 2021. Front Genet. 2021. PMID: 33777096 Free PMC article.
-
The Controversial Role of Polyploidy in Hepatocellular Carcinoma.Onco Targets Ther. 2021 Nov 27;14:5335-5344. doi: 10.2147/OTT.S340435. eCollection 2021. Onco Targets Ther. 2021. PMID: 34866913 Free PMC article. Review.
-
Impact of aging on primary liver cancer: epidemiology, pathogenesis and therapeutics.Aging (Albany NY). 2021 Oct 11;13(19):23416-23434. doi: 10.18632/aging.203620. Epub 2021 Oct 11. Aging (Albany NY). 2021. PMID: 34633987 Free PMC article. Review.
-
Transcriptomic Drivers of Differentiation, Maturation, and Polyploidy in Human Extravillous Trophoblast.Front Cell Dev Biol. 2021 Sep 3;9:702046. doi: 10.3389/fcell.2021.702046. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34540826 Free PMC article.
References
-
- Michalopoulos GK, DeFrances MC. Liver regeneration. Science 1997; 276: 60–66. - PubMed
-
- Si-Tayeb K, Lemaigre FP, Duncan SA. Organogenesis and development of the liver. Dev Cell 2010; 18: 175–189. - PubMed
-
- Jungermann K, Kietzmann T. Zonation of parenchymal and nonparenchymal metabolism in liver. Annu Rev Nutr 1996; 16: 179–203. - PubMed
-
- Bucher NLR, Malt RA Regeneration of Liver and Kidney. Little, Brown: Boston, MA, USA, 1971.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

