Oncolytic measles virus encoding interleukin-12 mediates potent antitumor effects through T cell activation
- PMID: 28507792
- PMCID: PMC5414860
- DOI: 10.1080/2162402X.2017.1285992
Oncolytic measles virus encoding interleukin-12 mediates potent antitumor effects through T cell activation
Abstract
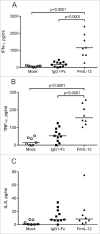
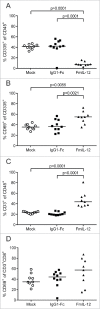
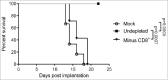
Combination of oncolytic virotherapy with immunomodulators is emerging as a promising therapeutic strategy for numerous tumor entities. In this study, we developed measles Schwarz vaccine strain vectors encoding immunomodulators to support different phases in the establishment of antitumor immune responses. Therapeutic efficacy of the novel vectors was evaluated in the immunocompetent MC38cea tumor model. We identified vectors encoding an IL-12 fusion protein (MeVac FmIL-12) and an antibody against PD-L1 (MeVac anti-PD-L1), respectively, as the most effective. Treatment of established tumors with MeVac FmIL-12 achieved 90% complete remissions. Profiling of the tumor immune microenvironment revealed activation of a type 1 T helper cell-directed response, with MeVac FmIL-12 ensuring potent early natural killer and effector T cell activation as well as upregulation of the effector cytokines IFN-γ and TNF-α. CD8+ T cells were found to be essential for the therapeutic efficacy of MeVac FmIL-12. Results of this study present MeVac FmIL-12 as a novel approach for targeted IL-12 delivery and elucidate mechanisms of successful immunovirotherapy.
Keywords: Anti-PD-L1; Oncolytic viruses; cancer immunotherapy; interleukin-12; measles virus.
Figures
Similar articles
-
Immunological Effects and Viral Gene Expression Determine the Efficacy of Oncolytic Measles Vaccines Encoding IL-12 or IL-15 Agonists.Viruses. 2019 Oct 3;11(10):914. doi: 10.3390/v11100914. Viruses. 2019. PMID: 31623390 Free PMC article.
-
Oncolytic Measles Virus Encoding Interleukin-12 Mediated Antitumor Activity and Immunologic Control of Colon Cancer In Vivo and Ex Vivo.Cancer Biother Radiopharm. 2021 Nov;36(9):774-782. doi: 10.1089/cbr.2019.3084. Epub 2020 Aug 12. Cancer Biother Radiopharm. 2021. PMID: 32783751
-
Oncolytic measles vaccines encoding PD-1 and PD-L1 checkpoint blocking antibodies to increase tumor-specific T cell memory.Mol Ther Oncolytics. 2021 Nov 29;24:43-58. doi: 10.1016/j.omto.2021.11.020. eCollection 2022 Mar 17. Mol Ther Oncolytics. 2021. PMID: 34977341 Free PMC article.
-
Emerging role of Natural killer cells in oncolytic virotherapy.Immunotargets Ther. 2015 Mar 31;4:65-77. doi: 10.2147/ITT.S55549. eCollection 2015. Immunotargets Ther. 2015. PMID: 27471713 Free PMC article. Review.
-
The oncolytic virus ΔPK has multimodal anti-tumor activity.Pathog Dis. 2016 Jul;74(5):ftw050. doi: 10.1093/femspd/ftw050. Epub 2016 May 29. Pathog Dis. 2016. PMID: 27242376 Free PMC article. Review.
Cited by
-
m6 A RNA methyltransferases METTL3/14 regulate immune responses to anti-PD-1 therapy.EMBO J. 2020 Oct 15;39(20):e104514. doi: 10.15252/embj.2020104514. Epub 2020 Sep 23. EMBO J. 2020. PMID: 32964498 Free PMC article.
-
Biomarker screen for efficacy of oncolytic virotherapy in patient-derived pancreatic cancer cultures.EBioMedicine. 2024 Jul;105:105219. doi: 10.1016/j.ebiom.2024.105219. Epub 2024 Jun 27. EBioMedicine. 2024. PMID: 38941955 Free PMC article.
-
Engineering strategies to enhance oncolytic viruses in cancer immunotherapy.Signal Transduct Target Ther. 2022 Apr 6;7(1):117. doi: 10.1038/s41392-022-00951-x. Signal Transduct Target Ther. 2022. PMID: 35387984 Free PMC article. Review.
-
Aptamers Enhance Oncolytic Viruses' Antitumor Efficacy.Pharmaceutics. 2022 Dec 31;15(1):151. doi: 10.3390/pharmaceutics15010151. Pharmaceutics. 2022. PMID: 36678780 Free PMC article. Review.
-
Oncolytic Virus Encoding a Master Pro-Inflammatory Cytokine Interleukin 12 in Cancer Immunotherapy.Cells. 2020 Feb 10;9(2):400. doi: 10.3390/cells9020400. Cells. 2020. PMID: 32050597 Free PMC article. Review.
References
-
- Bell J, McFadden G. Viruses for tumor therapy. Cell Host Microbe 2014; 15:260-5; PMID:24629333; http://dx.doi.org/10.1016/j.chom.2014.01.002 - DOI - PMC - PubMed
-
- Pol J, Buqué A, Aranda F, Bloy N, Cremer I, Eggermont A, Erbs P, Fucikova J, Galon J, Limacher JM et al.. Trial watch-oncolytic viruses and cancer therapy. Oncoimmunology 2016; 5:e1117740; PMID:27057469; http://dx.doi.org/10.1080/2162402X.2015.1117740 - DOI - PMC - PubMed
-
- Kaufman HL, Kohlhapp FJ, Zloza A. Oncolytic viruses: a new class of immunotherapy drugs. Nat Rev Drug Discov 2015; 14:642-62; PMID:26323545; http://dx.doi.org/10.1038/nrd4663 - DOI - PMC - PubMed
-
- Donnelly OG, Errington-Mais F, Steele L, Hadac E, Jennings V, Scott K, Peach H, Phillips RM, Bond J, Pandha H et al.. Measles virus causes immunogenic cell death in human melanoma. Gene Ther 2013; 20:7-15; PMID:22170342; http://dx.doi.org/10.1038/gt.2011.205 - DOI - PMC - PubMed
-
- Miyamoto S, Inoue H, Nakamura T, Yamada M, Sakamoto C, Urata Y, Okazaki T, Marumoto T, Takahashi A, Takayama K et al.. Coxsackievirus B3 is an oncolytic virus with immunostimulatory properties that is active against lung adenocarcinoma. Cancer Res 2012; 72:2609-21; PMID:22461509; http://dx.doi.org/10.1158/0008-5472.CAN-11-3185 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
