Phase II trial of the PI3 kinase inhibitor buparlisib (BKM-120) with or without enzalutamide in men with metastatic castration resistant prostate cancer
- PMID: 28502694
- PMCID: PMC6345641
- DOI: 10.1016/j.ejca.2017.02.030
Phase II trial of the PI3 kinase inhibitor buparlisib (BKM-120) with or without enzalutamide in men with metastatic castration resistant prostate cancer
Abstract
Background: Phosphatidylinositol-3-kinase (PI3K) and androgen receptor pathway activation is common in metastatic castration resistant prostate cancer (mCRPC). Buparlisib is an oral, pan-class I PI3 kinase inhibitor.
Methods: This was a multisite single arm phase II trial of buparlisib 100 mg ± enzalutamide daily in men with mCRPC whose disease progressed on or who were not candidates for docetaxel. The primary end-point was the rate of radiographic/clinical progression-free survival (PFS) at 6 months.
Results: Thirty men were accrued: 67% post-docetaxel; median prostate specific antigen (PSA) was 70 ng/dl, 83% had ≥4 prior therapies for mCRPC; 43% received concurrent enzalutamide. The final 6 month PFS rate was estimated to be 10% (95% confidence interval 2.5-23.6%). Median PFS was 1.9 months and was 3.5 months with concurrent enzalutamide. Median overall survival was 10.6 months. Concurrent enzalutamide led to a five-fold reduction in buparlisib concentrations. PSA declines were observed in 23%; no patients achieved a ≥50% decline, and no radiographic responses were observed. Severe adverse events occurred in four men including respiratory infection and multi-organ failure, urinary tract obstruction, confusion and one seizure in the setting of a new central nervous system (CNS) metastasis. Grade III adverse events were seen in 43% of patients; common toxicities included grade I-II weight loss, diarrhoea, nausea, fatigue, anorexia, rash, hyperglycemia and anxiety/mood disorders.
Conclusions: Buparlisib did not demonstrate significant activity in men with mCRPC, suggesting that PI3K inhibition is not sufficient to reverse resistant mCRPC progression. Future studies of PI3K pathway inhibitors with concurrent enzalutamide should develop optimal dosing and focus on selected patients more likely to benefit.
Keywords: BKM-120; Buparlisib; Clinical trial; Enzalutamide; Metastatic castration resistant prostate cancer; PI3 kinase; Prostate cancer.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Conflict of interest statement
The other authors report no potential conflicts of interest.
Figures
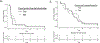
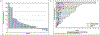
Comment in
-
Attaining precision therapy in prostate cancer: A tall order.Eur J Cancer. 2017 Aug;81:226-227. doi: 10.1016/j.ejca.2017.05.011. Epub 2017 Jun 16. Eur J Cancer. 2017. PMID: 28629596 Free PMC article. No abstract available.
Similar articles
-
[177Lu]Lu-PSMA-617 plus enzalutamide in patients with metastatic castration-resistant prostate cancer (ENZA-p): an open-label, multicentre, randomised, phase 2 trial.Lancet Oncol. 2024 May;25(5):563-571. doi: 10.1016/S1470-2045(24)00135-9. Epub 2024 Apr 12. Lancet Oncol. 2024. PMID: 38621400 Clinical Trial.
-
Bipolar androgen therapy in men with metastatic castration-resistant prostate cancer after progression on enzalutamide: an open-label, phase 2, multicohort study.Lancet Oncol. 2018 Jan;19(1):76-86. doi: 10.1016/S1470-2045(17)30906-3. Epub 2017 Dec 14. Lancet Oncol. 2018. PMID: 29248236 Free PMC article. Clinical Trial.
-
Clinical activity of enzalutamide in Docetaxel-naïve and Docetaxel-pretreated patients with metastatic castration-resistant prostate cancer.Prostate. 2014 Nov;74(15):1560-8. doi: 10.1002/pros.22874. Epub 2014 Aug 31. Prostate. 2014. PMID: 25176007 Free PMC article.
-
Enzalutamide for the treatment of nonmetastatic castration-resistant prostate cancer.Expert Opin Pharmacother. 2020 Dec;21(17):2091-2099. doi: 10.1080/14656566.2020.1803281. Epub 2020 Aug 12. Expert Opin Pharmacother. 2020. PMID: 32783772 Review.
-
Safety and effectiveness of enzalutamide in men with metastatic, castration-resistant prostate cancer.Expert Opin Pharmacother. 2015 Apr;16(5):749-54. doi: 10.1517/14656566.2015.1016911. Epub 2015 Feb 17. Expert Opin Pharmacother. 2015. PMID: 25687355 Review.
Cited by
-
Multiplex Immunofluorescence in Formalin-Fixed Paraffin-Embedded Tumor Tissue to Identify Single-Cell-Level PI3K Pathway Activation.Clin Cancer Res. 2020 Nov 15;26(22):5903-5913. doi: 10.1158/1078-0432.CCR-20-2000. Epub 2020 Sep 10. Clin Cancer Res. 2020. PMID: 32913135 Free PMC article.
-
Combination effect of therapies targeting the PI3K- and AR-signaling pathways in prostate cancer.Oncotarget. 2016 Nov 15;7(46):76181-76196. doi: 10.18632/oncotarget.12771. Oncotarget. 2016. PMID: 27783994 Free PMC article.
-
Reversal of Lactate and PD-1-mediated Macrophage Immunosuppression Controls Growth of PTEN/p53-deficient Prostate Cancer.Clin Cancer Res. 2023 May 15;29(10):1952-1968. doi: 10.1158/1078-0432.CCR-22-3350. Clin Cancer Res. 2023. PMID: 36862086 Free PMC article.
-
Targeting PI3K in cancer: mechanisms and advances in clinical trials.Mol Cancer. 2019 Feb 19;18(1):26. doi: 10.1186/s12943-019-0954-x. Mol Cancer. 2019. PMID: 30782187 Free PMC article. Review.
-
Suppression of tumor cell lactate-generating signaling pathways eradicates murine PTEN/p53-deficient aggressive-variant prostate cancer via macrophage phagocytosis.bioRxiv [Preprint]. 2023 May 23:2023.05.23.540590. doi: 10.1101/2023.05.23.540590. bioRxiv. 2023. Update in: Clin Cancer Res. 2023 Dec 1;29(23):4930-4940. doi: 10.1158/1078-0432.CCR-23-1441 PMID: 37292972 Free PMC article. Updated. Preprint.
References
-
- Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med 2010;363:411–22. - PubMed
-
- de Bono JS, Oudard S, Ozguroglu M, Hansen S, Machiels JP, Kocak I, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet 2010; 376:1147–54. - PubMed
-
- Parker C, Nilsson S, Heinrich D, O’Sullivan JM, Fossa SD, Chodacki A, et al. Updated analysis of the phase III, doubleblind, randomized, multinational study of radium-223 chloride in castration-resistant prostate cancer (CRPC) patients with bone metastases (ALSYMPCA). ASCO Meeting Abstracts, vol. 30;
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

