Hepatic uptake of conjugated bile acids is mediated by both sodium taurocholate cotransporting polypeptide and organic anion transporting polypeptides and modulated by intestinal sensing of plasma bile acid levels in mice
- PMID: 28498614
- PMCID: PMC5698707
- DOI: 10.1002/hep.29251
Hepatic uptake of conjugated bile acids is mediated by both sodium taurocholate cotransporting polypeptide and organic anion transporting polypeptides and modulated by intestinal sensing of plasma bile acid levels in mice
Abstract
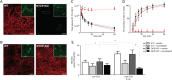
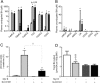
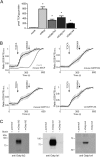
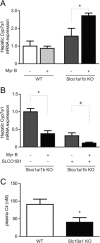
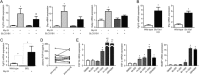
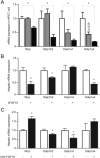
The Na+ -taurocholate cotransporting polypeptide (NTCP/SLC10A1) is believed to be pivotal for hepatic uptake of conjugated bile acids. However, plasma bile acid levels are normal in a subset of NTCP knockout mice and in mice treated with myrcludex B, a specific NTCP inhibitor. Here, we elucidated which transport proteins mediate the hepatic uptake of conjugated bile acids and demonstrated intestinal sensing of elevated bile acid levels in plasma in mice. Mice or healthy volunteers were treated with myrcludex B. Hepatic bile acid uptake kinetics were determined in wild-type (WT), organic anion transporting polypeptide (OATP) knockout mice (lacking Slco1a/1b isoforms), and human OATP1B1-transgenic mice. Effects of fibroblast growth factor 19 (FGF19) on hepatic transporter mRNA levels were assessed in rat hepatoma cells and in mice by peptide injection or adeno-associated virus-mediated overexpression. NTCP inhibition using myrcludex B had only moderate effects on bile acid kinetics in WT mice, but completely inhibited active transport of conjugated bile acid species in OATP knockout mice. Cholesterol 7α-hydroxylase Cyp7a1 expression was strongly down-regulated upon prolonged inhibition of hepatic uptake of conjugated bile acids. Fgf15 (mouse counterpart of FGF19) expression was induced in hypercholanemic OATP and NTCP knockout mice, as well as in myrcludex B-treated cholestatic mice, whereas plasma FGF19 was not induced in humans treated with myrcludex B. Fgf15/FGF19 expression was induced in polarized human enterocyte-models and mouse organoids by basolateral incubation with a high concentration (1 mM) of conjugated bile acids.
Conclusion: NTCP and OATPs contribute to hepatic uptake of conjugated bile acids in mice, whereas the predominant uptake in humans is NTCP mediated. Enterocytes sense highly elevated levels of (conjugated) bile acids in the systemic circulation to induce FGF15/19, which modulates hepatic bile acid synthesis and uptake. (Hepatology 2017;66:1631-1643).
© 2017 The Authors. Hepatology published by Wiley Periodicals, Inc., on behalf of the American Association for the Study of Liver Diseases.
Figures
Comment in
-
Hepatic bile acid uptake in humans and mice: Multiple pathways and expanding potential role for gut-liver signaling.Hepatology. 2017 Nov;66(5):1384-1386. doi: 10.1002/hep.29325. Epub 2017 Sep 29. Hepatology. 2017. PMID: 28646543 Free PMC article. No abstract available.
-
The role of microsomal epoxide hydrolase, Na+ -taurocholate cotransporting polypeptide, and organic anion transporting polypeptide in hepatic sodium-dependent bile acid transport.Hepatology. 2018 Mar;67(3):1184-1185. doi: 10.1002/hep.29712. Epub 2018 Jan 30. Hepatology. 2018. PMID: 29211931 No abstract available.
-
Reply.Hepatology. 2018 Mar;67(3):1185-1186. doi: 10.1002/hep.29709. Epub 2018 Jan 30. Hepatology. 2018. PMID: 29211937 No abstract available.
Similar articles
-
Inhibition of Hepatic Bile Acid Uptake by Myrcludex B Promotes Glucagon-Like Peptide-1 Release and Reduces Obesity.Cell Mol Gastroenterol Hepatol. 2020;10(3):451-466. doi: 10.1016/j.jcmgh.2020.04.009. Epub 2020 Apr 21. Cell Mol Gastroenterol Hepatol. 2020. PMID: 32330730 Free PMC article.
-
Impaired uptake of conjugated bile acids and hepatitis b virus pres1-binding in na(+) -taurocholate cotransporting polypeptide knockout mice.Hepatology. 2015 Jul;62(1):207-19. doi: 10.1002/hep.27694. Epub 2015 May 8. Hepatology. 2015. PMID: 25641256 Free PMC article.
-
Inhibition of ileal apical but not basolateral bile acid transport reduces atherosclerosis in apoE⁻/⁻ mice.Atherosclerosis. 2013 Aug;229(2):374-80. doi: 10.1016/j.atherosclerosis.2013.05.017. Epub 2013 May 28. Atherosclerosis. 2013. PMID: 23880190 Free PMC article.
-
Molecular regulation of the hepatic bile acid uptake transporter and HBV entry receptor NTCP.Biochim Biophys Acta Mol Cell Biol Lipids. 2021 Aug;1866(8):158960. doi: 10.1016/j.bbalip.2021.158960. Epub 2021 Apr 29. Biochim Biophys Acta Mol Cell Biol Lipids. 2021. PMID: 33932583 Review.
-
Molecular regulation of sinusoidal liver bile acid transporters during cholestasis.Yale J Biol Med. 1997 Jul-Aug;70(4):355-63. Yale J Biol Med. 1997. PMID: 9626756 Free PMC article. Review.
Cited by
-
Increased sulfation of bile acids in mice and human subjects with sodium taurocholate cotransporting polypeptide deficiency.J Biol Chem. 2019 Aug 2;294(31):11853-11862. doi: 10.1074/jbc.RA118.007179. Epub 2019 Jun 14. J Biol Chem. 2019. PMID: 31201272 Free PMC article.
-
Inhibition of Hepatic Bile Acid Uptake by Myrcludex B Promotes Glucagon-Like Peptide-1 Release and Reduces Obesity.Cell Mol Gastroenterol Hepatol. 2020;10(3):451-466. doi: 10.1016/j.jcmgh.2020.04.009. Epub 2020 Apr 21. Cell Mol Gastroenterol Hepatol. 2020. PMID: 32330730 Free PMC article.
-
Na+ -taurocholate cotransporting polypeptide inhibition has hepatoprotective effects in cholestasis in mice.Hepatology. 2018 Sep;68(3):1057-1069. doi: 10.1002/hep.29888. Epub 2018 Apr 27. Hepatology. 2018. PMID: 29572910 Free PMC article.
-
NTCP deficiency in mice protects against obesity and hepatosteatosis.JCI Insight. 2019 Jun 25;5(14):e127197. doi: 10.1172/jci.insight.127197. JCI Insight. 2019. PMID: 31237863 Free PMC article.
-
Characterization of organic anion transporting polypeptide 1b2 knockout rats generated by CRISPR/Cas9: a novel model for drug transport and hyperbilirubinemia disease.Acta Pharm Sin B. 2020 May;10(5):850-860. doi: 10.1016/j.apsb.2019.11.007. Epub 2019 Nov 14. Acta Pharm Sin B. 2020. PMID: 32528832 Free PMC article.
References
-
- Thomas C, Pellicciari R, Pruzanski M, Auwerx J, Schoonjans K. Targeting bile‐acid signalling for metabolic diseases. Nat Rev Drug Discov 2008;7:678‐693. - PubMed
-
- Lutgehetmann M, Mancke LV, Volz T, Helbig M, Allweiss L, Bornscheuer T, et al. Humanized chimeric uPA mouse model for the study of hepatitis B and D virus interactions and preclinical drug evaluation. Hepatology 2012;55:685‐694. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

