HCMV activation of ERK-MAPK drives a multi-factorial response promoting the survival of infected myeloid progenitors
- PMID: 28491825
- PMCID: PMC5421601
HCMV activation of ERK-MAPK drives a multi-factorial response promoting the survival of infected myeloid progenitors
Abstract
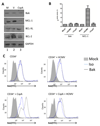
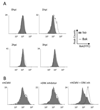
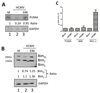
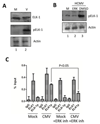
Viral binding and entry provides the first trigger of a cell death response and thus how human cytomegalovirus (HCMV) evades this - particularly during latent infection where a very limited pattern of gene expression is observed - is less well understood. It has been demonstrated that the activation of cellular signalling pathways upon virus binding promotes the survival of latently infected cells by the activation of cell encoded anti-apoptotic responses. In CD34+ cells, a major site of HCMV latency, ERK signalling is important for survival and we now show that the activation of this pathway impacts on multiple aspects of cell death pathways. The data illustrate that HCMV infection triggers activation of pro-apoptotic Bak which is then countered through multiple ERK-dependent functions. Specifically, ERK promotes ELK1 mediated transcription of the key survival molecule MCL-1, along with a concomitant decrease of the pro-apoptotic BIM and PUMA proteins. Finally, we show that the elimination of ELK-1 from CD34+ cells results in elevated Bak activation in response to viral infection, resulting in cell death. Taken together, these data begin to shed light on the poly-functional response elicited by HCMV via ERK-MAPK to promote cell survival.
Conflict of interest statement
Conflicts of interest The authors declare no conflicts of interest associated with this work.
Figures
Similar articles
-
Human cytomegalovirus activation of ERK and myeloid cell leukemia-1 protein correlates with survival of latently infected cells.Proc Natl Acad Sci U S A. 2012 Jan 10;109(2):588-93. doi: 10.1073/pnas.1114966108. Epub 2011 Dec 27. Proc Natl Acad Sci U S A. 2012. PMID: 22203987 Free PMC article.
-
Latency-Associated Expression of Human Cytomegalovirus US28 Attenuates Cell Signaling Pathways To Maintain Latent Infection.mBio. 2017 Dec 5;8(6):e01754-17. doi: 10.1128/mBio.01754-17. mBio. 2017. PMID: 29208743 Free PMC article.
-
Human Cytomegalovirus Stimulates the Synthesis of Select Akt-Dependent Antiapoptotic Proteins during Viral Entry To Promote Survival of Infected Monocytes.J Virol. 2016 Jan 6;90(6):3138-47. doi: 10.1128/JVI.02879-15. J Virol. 2016. PMID: 26739047 Free PMC article.
-
Chromatin-mediated regulation of cytomegalovirus gene expression.Virus Res. 2011 May;157(2):134-43. doi: 10.1016/j.virusres.2010.09.019. Epub 2010 Sep 25. Virus Res. 2011. PMID: 20875471 Free PMC article. Review.
-
Aspects of human cytomegalovirus latency and reactivation.Curr Top Microbiol Immunol. 2008;325:297-313. doi: 10.1007/978-3-540-77349-8_17. Curr Top Microbiol Immunol. 2008. PMID: 18637513 Review.
Cited by
-
Host signaling and EGR1 transcriptional control of human cytomegalovirus replication and latency.PLoS Pathog. 2019 Nov 14;15(11):e1008037. doi: 10.1371/journal.ppat.1008037. eCollection 2019 Nov. PLoS Pathog. 2019. PMID: 31725811 Free PMC article.
-
The Role of the Human Cytomegalovirus UL133-UL138 Gene Locus in Latency and Reactivation.Viruses. 2020 Jul 1;12(7):714. doi: 10.3390/v12070714. Viruses. 2020. PMID: 32630219 Free PMC article. Review.
-
Role of the ERK1/2 Signaling Pathway in the Replication of Junín and Tacaribe Viruses.Viruses. 2018 Apr 17;10(4):199. doi: 10.3390/v10040199. Viruses. 2018. PMID: 29673133 Free PMC article.
-
Cytomegalovirus Latency and Reactivation: An Intricate Interplay With the Host Immune Response.Front Cell Infect Microbiol. 2020 Mar 31;10:130. doi: 10.3389/fcimb.2020.00130. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 32296651 Free PMC article. Review.
-
Prognostic Value and Related Regulatory Networks of MRPL15 in Non-Small-Cell Lung Cancer.Front Oncol. 2021 May 7;11:656172. doi: 10.3389/fonc.2021.656172. eCollection 2021. Front Oncol. 2021. PMID: 34026630 Free PMC article.
References
-
- Barry MA, Behnke CA, Eastman A. Activation of programmed cell death (apoptosis) by cisplatin, other anticancer drugs, toxins and hyperthermia. Biochem Pharmacol. 1990;40:2353–2362. - PubMed
-
- Bieniasz PD. Intrinsic immunity: a front-line defense against viral attack. Nat Immunol. 2004;5:1109–1115. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
