Myeloid-derived miR-223 regulates intestinal inflammation via repression of the NLRP3 inflammasome
- PMID: 28487310
- PMCID: PMC5460990
- DOI: 10.1084/jem.20160462
Myeloid-derived miR-223 regulates intestinal inflammation via repression of the NLRP3 inflammasome
Abstract
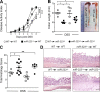
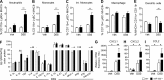
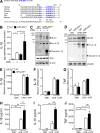
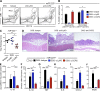
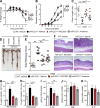
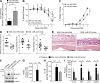
MicroRNA (miRNA)-mediated RNA interference regulates many immune processes, but how miRNA circuits orchestrate aberrant intestinal inflammation during inflammatory bowel disease (IBD) is poorly defined. Here, we report that miR-223 limits intestinal inflammation by constraining the nlrp3 inflammasome. miR-223 was increased in intestinal biopsies from patients with active IBD and in preclinical models of intestinal inflammation. miR-223-/y mice presented with exacerbated myeloid-driven experimental colitis with heightened clinical, histopathological, and cytokine readouts. Mechanistically, enhanced NLRP3 inflammasome expression with elevated IL-1β was a predominant feature during the initiation of colitis with miR-223 deficiency. Depletion of CCR2+ inflammatory monocytes and pharmacologic blockade of IL-1β or NLRP3 abrogated this phenotype. Generation of a novel mouse line, with deletion of the miR-223 binding site in the NLRP3 3' untranslated region, phenocopied the characteristics of miR-223-/y mice. Finally, nanoparticle-mediated overexpression of miR-223 attenuated experimental colitis, NLRP3 levels, and IL-1β release. Collectively, our data reveal a previously unappreciated role for miR-223 in regulating the innate immune response during intestinal inflammation.
© 2017 Neudecker et al.
Figures


Similar articles
-
Protective and aggravating effects of Nlrp3 inflammasome activation in IBD models: influence of genetic and environmental factors.Dig Dis. 2012;30 Suppl 1:82-90. doi: 10.1159/000341681. Epub 2012 Oct 11. Dig Dis. 2012. PMID: 23075874
-
MicroRNA-495 Ameliorates Cardiac Microvascular Endothelial Cell Injury and Inflammatory Reaction by Suppressing the NLRP3 Inflammasome Signaling Pathway.Cell Physiol Biochem. 2018;49(2):798-815. doi: 10.1159/000493042. Epub 2018 Aug 30. Cell Physiol Biochem. 2018. PMID: 30165354
-
MiR-223 plays a protecting role in neutrophilic asthmatic mice through the inhibition of NLRP3 inflammasome.Respir Res. 2020 May 18;21(1):116. doi: 10.1186/s12931-020-01374-4. Respir Res. 2020. PMID: 32423405 Free PMC article.
-
Inflammasome Regulation: Therapeutic Potential for Inflammatory Bowel Disease.Molecules. 2021 Mar 19;26(6):1725. doi: 10.3390/molecules26061725. Molecules. 2021. PMID: 33808793 Free PMC article. Review.
-
Targeting NLRP3 Inflammasome in Inflammatory Bowel Disease: Putting out the Fire of Inflammation.Inflammation. 2019 Aug;42(4):1147-1159. doi: 10.1007/s10753-019-01008-y. Inflammation. 2019. PMID: 30937839 Review.
Cited by
-
A Role for Folate in Microbiome-Linked Control of Autoimmunity.J Immunol Res. 2021 May 19;2021:9998200. doi: 10.1155/2021/9998200. eCollection 2021. J Immunol Res. 2021. PMID: 34104654 Free PMC article. Review.
-
NLRP3 inflammasome in digestive diseases: From mechanism to therapy.Front Immunol. 2022 Oct 26;13:978190. doi: 10.3389/fimmu.2022.978190. eCollection 2022. Front Immunol. 2022. PMID: 36389791 Free PMC article. Review.
-
Progressive Control of Streptococcus agalactiae-Induced Innate Inflammatory Response Is Associated with Time Course Expression of MicroRNA-223 by Neutrophils.Infect Immun. 2020 Nov 16;88(12):e00563-20. doi: 10.1128/IAI.00563-20. Print 2020 Nov 16. Infect Immun. 2020. PMID: 32958526 Free PMC article.
-
Expression and Clinical Significance of Plasma miR-223 in Patients with Diabetic Nephropathy.Int J Endocrinol. 2023 Dec 27;2023:9663320. doi: 10.1155/2023/9663320. eCollection 2023. Int J Endocrinol. 2023. PMID: 38179188 Free PMC article.
-
A potential role of microvesicle-containing miR-223/142 in lung inflammation.Thorax. 2019 Sep;74(9):865-874. doi: 10.1136/thoraxjnl-2018-212994. Epub 2019 Jul 22. Thorax. 2019. PMID: 31331947 Free PMC article.
References
-
- Allen I.C., TeKippe E.M., Woodford R.M., Uronis J.M., Holl E.K., Rogers A.B., Herfarth H.H., Jobin C., and Ting J.P.. 2010. The NLRP3 inflammasome functions as a negative regulator of tumorigenesis during colitis-associated cancer. J. Exp. Med. 207:1045–1056. 10.1084/jem.20100050 - DOI - PMC - PubMed
-
- Andres P.G., Beck P.L., Mizoguchi E., Mizoguchi A., Bhan A.K., Dawson T., Kuziel W.A., Maeda N., MacDermott R.P., Podolsky D.K., and Reinecker H.C.. 2000. Mice with a selective deletion of the CC chemokine receptors 5 or 2 are protected from dextran sodium sulfate-mediated colitis: Lack of CC chemokine receptor 5 expression results in a NK1.1+ lymphocyte-associated Th2-type immune response in the intestine. J. Immunol. 164:6303–6312. 10.4049/jimmunol.164.12.6303 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

