Endoplasmic Reticulum Stress-Induced CHOP Inhibits PGC-1α and Causes Mitochondrial Dysfunction in Diabetic Embryopathy
- PMID: 28482072
- PMCID: PMC5837255
- DOI: 10.1093/toxsci/kfx096
Endoplasmic Reticulum Stress-Induced CHOP Inhibits PGC-1α and Causes Mitochondrial Dysfunction in Diabetic Embryopathy
Abstract
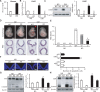
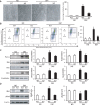
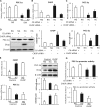
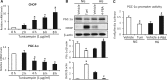
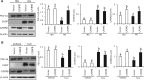
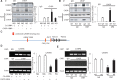
Endoplasmic reticulum (ER) stress has been implicated in the development of maternal diabetes-induced neural tube defects (NTDs). ER stress-induced C/EBP homologous protein (CHOP) plays an important role in the pro-apoptotic execution pathways. However, the molecular mechanism underlying ER stress- and CHOP-induced neuroepithelium cell apoptosis in diabetic embryopathy is still unclear. Deletion of the Chop gene significantly reduced maternal diabetes-induced NTDs. CHOP deficiency abrogated maternal diabetes-induced mitochondrial dysfunction and neuroepithelium cell apoptosis. Further analysis demonstrated that CHOP repressed the expression of peroxisome-proliferator-activated receptor-γ coactivator-1α (PGC-1α), an essential regulator for mitochondrial biogenesis and function. Both CHOP deficiency in vivo and knockdown in vitro restore high glucose-suppressed PGC-1α expression. In contrast, CHOP overexpression mimicked inhibition of PGC-1α by high glucose. In response to the ER stress inducer tunicamycin, PGC-1α expression was decreased, whereas the ER stress inhibitor 4-phenylbutyric acid blocked high glucose-suppressed PGC-1α expression. Moreover, maternal diabetes in vivo and high glucose in vitro promoted the interaction between CHOP and the PGC-1α transcriptional regulator CCAAT/enhancer binding protein-β (C/EBPβ), and reduced C/EBPβ binding to the PGC-1α promoter leading to markedly decrease in PGC-1α expression. Together, our findings support the hypothesis that maternal diabetes-induced ER stress increases CHOP expression which represses PGC-1α through suppressing the C/EBPβ transcriptional activity, subsequently induces mitochondrial dysfunction and ultimately results in NTDs.
Keywords: C/EBPβ; CHOP; ER stress; PGC-1α; diabetic embryopathy; mitochondrial dysfunction; neural tube defects.
© The Author 2017. Published by Oxford University Press on behalf of the Society of Toxicology. All rights reserved. For Permissions, please e-mail: journals.permissions@oup.com.
Figures
Similar articles
-
Silenced CHOP protects pancreatic B-cell function by targeting peroxisome proliferator-activated receptor-γ coactivator-1α through nuclear factor-κB signaling pathway in diabetes mellitus.J Cell Biochem. 2019 Aug;120(8):12595-12603. doi: 10.1002/jcb.28526. Epub 2019 Mar 8. J Cell Biochem. 2019. PMID: 30848505
-
Superoxide dismutase 2 overexpression alleviates maternal diabetes-induced neural tube defects, restores mitochondrial function and suppresses cellular stress in diabetic embryopathy.Free Radic Biol Med. 2016 Jul;96:234-44. doi: 10.1016/j.freeradbiomed.2016.04.030. Epub 2016 Apr 27. Free Radic Biol Med. 2016. PMID: 27130031 Free PMC article.
-
Deficiency of the oxidative stress-responsive kinase p70S6K1 restores autophagy and ameliorates neural tube defects in diabetic embryopathy.Am J Obstet Gynecol. 2020 Nov;223(5):753.e1-753.e14. doi: 10.1016/j.ajog.2020.05.015. Epub 2020 May 13. Am J Obstet Gynecol. 2020. PMID: 32416155 Free PMC article.
-
Decoding the oxidative stress hypothesis in diabetic embryopathy through proapoptotic kinase signaling.Am J Obstet Gynecol. 2015 May;212(5):569-79. doi: 10.1016/j.ajog.2014.11.036. Epub 2014 Nov 27. Am J Obstet Gynecol. 2015. PMID: 25434839 Free PMC article. Review.
-
PGC-1α, a potential therapeutic target against kidney aging.Aging Cell. 2019 Oct;18(5):e12994. doi: 10.1111/acel.12994. Epub 2019 Jul 16. Aging Cell. 2019. PMID: 31313501 Free PMC article. Review.
Cited by
-
Embryonic diapause due to high glucose is related to changes in glycolysis and oxidative phosphorylation, as well as abnormalities in the TCA cycle and amino acid metabolism.Front Endocrinol (Lausanne). 2023 Dec 18;14:1135837. doi: 10.3389/fendo.2023.1135837. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 38170036 Free PMC article.
-
Peroxisome proliferator-activated receptor gamma coactivator-1 (PGC-1) family in physiological and pathophysiological process and diseases.Signal Transduct Target Ther. 2024 Mar 1;9(1):50. doi: 10.1038/s41392-024-01756-w. Signal Transduct Target Ther. 2024. PMID: 38424050 Free PMC article. Review.
-
Roles of mitochondrial unfolded protein response in mammalian stem cells.World J Stem Cells. 2021 Jul 26;13(7):737-752. doi: 10.4252/wjsc.v13.i7.737. World J Stem Cells. 2021. PMID: 34367475 Free PMC article. Review.
-
Bhlhe40/Sirt1 Axis-Regulated Mitophagy Is Implicated in All-Trans Retinoic Acid-Induced Spina Bifida Aperta.Front Cell Dev Biol. 2021 Apr 27;9:644346. doi: 10.3389/fcell.2021.644346. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33987177 Free PMC article.
-
TLR9 Signaling Protects Alcohol-Induced Hepatic Oxidative Stress but Worsens Liver Inflammation in Mice.Front Pharmacol. 2021 Jun 28;12:709002. doi: 10.3389/fphar.2021.709002. eCollection 2021. Front Pharmacol. 2021. PMID: 34262465 Free PMC article.
References
-
- Arany Z., He H., Lin J., Hoyer K., Handschin C., Toka O., Ahmad F., Matsui T., Chin S., Wu P. H., et al. (2005). Transcriptional coactivator PGC-1 alpha controls the energy state and contractile function of cardiac muscle. Cell Metab. 1, 259–271. - PubMed
-
- Austin S., St-Pierre J. (2012). PGC1alpha and mitochondrial metabolism–emerging concepts and relevance in ageing and neurodegenerative disorders. J. Cell Sci. 125, 4963–4971. - PubMed
-
- De Bellard M. E., Ching W., Gossler A., Bronner-Fraser M. (2002). Disruption of segmental neural crest migration and ephrin expression in delta-1 null mice. Dev. Biol. 249, 121–130. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

