Ultrastructural and biochemical aspects of matrix vesicle-mediated mineralization
- PMID: 28479934
- PMCID: PMC5405202
- DOI: 10.1016/j.jdsr.2016.09.002
Ultrastructural and biochemical aspects of matrix vesicle-mediated mineralization
Abstract
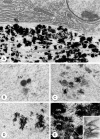
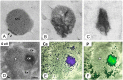
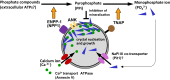
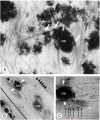
Matrix vesicle-mediated mineralization is an orchestrated sequence of ultrastructural and biochemical events that lead to crystal nucleation and growth. The influx of phosphate ions into the matrix vesicle is mediated by several proteins such as TNAP, ENPP1, Pit1, annexin and so forth. The catalytic activity of ENPP1 generates pyrophosphate (PPi) using extracellular ATPs as a substrate, and the resultant PPi prevents crystal overgrowth. However, TNAP hydrolyzes PPi into phosphate ion monomers, which are then transported into the matrix vesicle through Pit1. Accumulation of Ca2+ and PO43- inside matrix vesicles then induces crystalline nucleation, with calcium phosphate crystals budding off radially, puncturing the matrix vesicle's membrane and finally growing out of it to form mineralized nodules.
Keywords: ENPP1; Matrix vesicle; Mineralization; Mineralized nodule; TNAP.
Figures




Similar articles
-
Ultrastructure and biological function of matrix vesicles in bone mineralization.Histochem Cell Biol. 2018 Apr;149(4):289-304. doi: 10.1007/s00418-018-1646-0. Epub 2018 Feb 6. Histochem Cell Biol. 2018. PMID: 29411103 Review.
-
Matrix Vesicle-Mediated Mineralization and Osteocytic Regulation of Bone Mineralization.Int J Mol Sci. 2022 Sep 1;23(17):9941. doi: 10.3390/ijms23179941. Int J Mol Sci. 2022. PMID: 36077336 Free PMC article. Review.
-
[Updates on rickets and osteomalacia: mechanism and regulation of bone mineralization].Clin Calcium. 2013 Oct;23(10):1463-7. Clin Calcium. 2013. PMID: 24076644 Review. Japanese.
-
[Microscopic aspects on biomineralization in bone].Clin Calcium. 2014 Feb;24(2):203-14. Clin Calcium. 2014. PMID: 24473353 Review. Japanese.
-
Nature of phosphate substrate as a major determinant of mineral type formed in matrix vesicle-mediated in vitro mineralization: An FTIR imaging study.Bone. 2006 Jun;38(6):811-7. doi: 10.1016/j.bone.2005.11.027. Epub 2006 Feb 3. Bone. 2006. PMID: 16461032
Cited by
-
Matrix Vesicles: Role in Bone Mineralization and Potential Use as Therapeutics.Pharmaceuticals (Basel). 2021 Mar 24;14(4):289. doi: 10.3390/ph14040289. Pharmaceuticals (Basel). 2021. PMID: 33805145 Free PMC article. Review.
-
Raman spectroscopy links differentiating osteoblast matrix signatures to pro-angiogenic potential.Matrix Biol Plus. 2019 Nov 20;5:100018. doi: 10.1016/j.mbplus.2019.100018. eCollection 2020 Feb. Matrix Biol Plus. 2019. PMID: 33543015 Free PMC article.
-
Experimental Early Stimulation of Bone Tissue Neo-Formation for Critical Size Elimination Defects in the Maxillofacial Region.Polymers (Basel). 2023 Oct 26;15(21):4232. doi: 10.3390/polym15214232. Polymers (Basel). 2023. PMID: 37959911 Free PMC article.
-
Morphological Reconstruction of a Critical-Sized Bone Defect in the Maxillofacial Region Using Modified Chitosan in Rats with Sub-Compensated Type I Diabetes Mellitus.Polymers (Basel). 2023 Nov 6;15(21):4337. doi: 10.3390/polym15214337. Polymers (Basel). 2023. PMID: 37960017 Free PMC article.
-
Autophagic LC3+ calcified extracellular vesicles initiate cartilage calcification in osteoarthritis.Sci Adv. 2022 May 13;8(19):eabn1556. doi: 10.1126/sciadv.abn1556. Epub 2022 May 11. Sci Adv. 2022. PMID: 35544558 Free PMC article.
References
-
- Bonucci E. Fine structure of early cartilage calcification. J Ultrastruct Res. 1967;20:33–50. - PubMed
-
- Bonucci E. Fine structure and histochemistry of “calcifying globules” in epiphyseal cartilage. Z Zellforsch Mikrosk Anat. 1970;103:192–217. - PubMed
-
- Ozawa H., Yamada M., Yajima T. The ultrastructural and cytochemical aspects of matrix vesicles and calcification processes. In: Talmage R.V., Ozawa H., editors. Formation and calcification of hard tissues. Shakai Hoken Pub; Tokyo: 1978. pp. 9–57.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

