Concomitant Disruption of CD4 and CD8 Genes Facilitates the Development of Double Negative αβ TCR+ Peripheral T Cells That Respond Robustly to Staphylococcal Superantigen
- PMID: 28468970
- PMCID: PMC5471834
- DOI: 10.4049/jimmunol.1601991
Concomitant Disruption of CD4 and CD8 Genes Facilitates the Development of Double Negative αβ TCR+ Peripheral T Cells That Respond Robustly to Staphylococcal Superantigen
Abstract
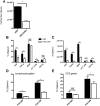
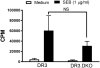
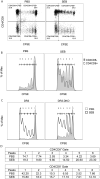
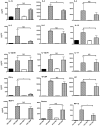
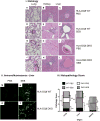
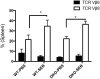
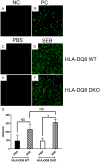
Mature peripheral double negative T (DNT) cells expressing αβ TCR but lacking CD4/CD8 coreceptors play protective as well as pathogenic roles. To better understand their development and functioning in vivo, we concomitantly inactivated CD4 and CD8 genes in mice with intact MHC class I and class II molecules with the hypothesis that this would enable the development of DNT cells. We also envisaged that these DNT cells could be activated by bacterial superantigens in vivo as activation of T cells by superantigens does not require CD4 and CD8 coreceptors. Because HLA class II molecules present superantigens more efficiently than murine MHC class II molecules, CD4 CD8 double knockout (DKO) mice transgenically expressing HLA-DR3 or HLA-DQ8 molecules were generated. Although thymic cellularity was comparable between wild type (WT) and DKO mice, CD3+ αβ TCR+ thymocytes were significantly reduced in DKO mice, implying defects in thymic-positive selection. Splenic CD3+ αβ TCR+ cells and Foxp3+ T regulatory cells were present in DKO mice but significantly reduced. However, the in vivo inflammatory responses and immunopathology elicited by acute challenge with the staphylococcal superantigen enterotoxin B were comparable between WT and DKO mice. Choric exposure to staphylococcal enterotoxin B precipitated a lupus-like inflammatory disease with characteristic lympho-monocytic infiltration in lungs, livers, and kidneys, along with production of anti-nuclear Abs in DKO mice as in WT mice. Overall, our results suggest that DNT cells can develop efficiently in vivo and chronic exposure to bacterial superantigens may precipitate a lupus-like autoimmune disease through activation of DNT cells.
Copyright © 2017 by The American Association of Immunologists, Inc.
Figures
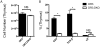
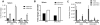

Similar articles
-
Self-specific MHC class II-restricted CD4-CD8- T cells that escape deletion and lack regulatory activity.J Immunol. 2003 Jan 1;170(1):201-9. doi: 10.4049/jimmunol.170.1.201. J Immunol. 2003. PMID: 12496401
-
Expression and function of HLA-DR3 and DQ8 in transgenic mice lacking functional H2-M.Tissue Antigens. 2003 Aug;62(2):149-61. doi: 10.1034/j.1399-0039.2003.00088.x. Tissue Antigens. 2003. PMID: 12889995
-
In vivo staphylococcal superantigen-driven polyclonal Ig responses in mice: dependence upon CD4(+) cells and human MHC class II.Int Immunol. 2001 Oct;13(10):1291-300. doi: 10.1093/intimm/13.10.1291. Int Immunol. 2001. PMID: 11581174
-
Thymic repertoire selection by superantigens: presentation by human and mouse MHC molecules.Thymus. 1994;23(1):1-13. Thymus. 1994. PMID: 7863543 Review.
-
The CD8 T cell response to staphylococcal enterotoxins.Semin Immunol. 1993 Feb;5(1):33-9. doi: 10.1006/smim.1993.1005. Semin Immunol. 1993. PMID: 8467093 Review.
Cited by
-
NKG2D Enhances Double-Negative T Cell Regulation of B Cells.Front Immunol. 2021 Jun 16;12:650788. doi: 10.3389/fimmu.2021.650788. eCollection 2021. Front Immunol. 2021. PMID: 34220808 Free PMC article.
-
DNT cells mediate resistance to CAR-T cells therapy in a pediatric patient with relapsed and refractory B-ALL.Ann Hematol. 2024 Jul;103(7):2551-2556. doi: 10.1007/s00277-024-05790-0. Epub 2024 May 10. Ann Hematol. 2024. PMID: 38724656
-
Expansion of Double-Negative T Cells in Patients before Liver Transplantation Correlates with Post-Transplant Infections.J Clin Med. 2022 Jun 17;11(12):3502. doi: 10.3390/jcm11123502. J Clin Med. 2022. PMID: 35743569 Free PMC article.
-
Double-negative T cells: a promising avenue of adoptive cell therapy in transplant oncology.J Zhejiang Univ Sci B. 2023 May 15;24(5):387-396. doi: 10.1631/jzus.B2200528. J Zhejiang Univ Sci B. 2023. PMID: 37190888 Free PMC article.
References
-
- Egawa T. Chapter One - Regulation of CD4 and CD8 Coreceptor Expression and CD4 Versus CD8 Lineage Decisions. In: Frederick WA, editor. Advances in Immunology. Academic Press; 2015. pp. 1–40. - PubMed
-
- Singer A, Bosselut R. Advances in Immunology. Academic Press; 2004. CD4/CD8 Coreceptors in Thymocyte Development, Selection, and Lineage Commitment: Analysis of the CD4/CD8 Lineage Decision; pp. 91–131. - PubMed
-
- Alberts B, Johnson A, Lewis J, et al. Helper T Cells and Lymphocyte Activation 2002
-
- Andersen MH, Schrama D, thor Straten P, Becker JC. Cytotoxic T Cells. Journal of Investigative Dermatology. 2006;126:32–41. - PubMed
-
- Fischer K, Voelkl S, Heymann J, Przybylski GK, Mondal K, Laumer M, Kunz-Schughart L, Schmidt CA, Andreesen R, Mackensen A. Isolation and characterization of human antigen-specific TCRαβ+CD4-CD8- double-negative regulatory T cells. Blood. 2005;105:2828. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

