Immunotherapy with single agent nivolumab for advanced leiomyosarcoma of the uterus: Results of a phase 2 study
- PMID: 28440953
- PMCID: PMC5762200
- DOI: 10.1002/cncr.30738
Immunotherapy with single agent nivolumab for advanced leiomyosarcoma of the uterus: Results of a phase 2 study
Abstract
Background: Immunotherapy has changed the therapeutic landscape in oncology. Advanced uterine leiomyosarcoma (ULMS) remains an incurable disease in most cases, and despite new drug approvals, improvements in overall survival have been modest at best. The goal of this study was to evaluate programmed-death 1 (PD-1) inhibition with nivolumab in this patient population.
Methods: This single-center phase 2 trial completed enrollment between May and October 2015. Patients received 3 mg/kg of intravenous nivolumab on day 1 of each 2-week cycle until disease progression or unacceptable toxicity. The primary endpoint was objective response rate. We assessed PD-1, PD-ligand 1 (PD-L1), and PD-L2 expression in archival tumor samples and variations in immune-phenotyping of circulating immune cells during treatment.
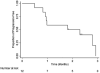
Results: Twelve patients were enrolled in the first stage of the 2-stage design. A median of 5 (range, 2-6) 2-week cycles of nivolumab were administered. Of the 12 patients, none responded to treatment. The overall median progression-free survival was 1.8 months (95% confidence interval, 0.8-unknown). The study did not open the second stage due to lack of benefit as defined by the statistical plan. Archival samples were available for 83% of patients. PD-1 (>3% of cells), PD-L1, and PD-L2 (>5% and >10% of tumor cells, respectively) expression were observed in 20%, 20%, and 90% of samples, respectively. No significant differences were observed between pre- and posttreatment cell phenotypes.
Conclusion: Single-agent nivolumab did not demonstrate a benefit in this cohort of previously treated advanced ULMS patients. Further biomarker-driven approaches and studies evaluating combined immune checkpoint-modulators should be considered. Cancer 2017;123:3285-90. © 2017 American Cancer Society.
Keywords: anti-programmed-death 1 (PD-1); immunotherapy; nivolumab; sarcoma; uterine leiomyosarcoma.
© 2017 American Cancer Society.
Figures
Similar articles
-
The influence of primary site on outcomes in leiomyosarcoma: a review of clinicopathologic differences between uterine and extrauterine disease.Am J Clin Oncol. 2013 Aug;36(4):368-74. doi: 10.1097/COC.0b013e318248dbf4. Am J Clin Oncol. 2013. PMID: 22772425
-
Efficacy and safety of nivolumab in Japanese patients with uterine cervical cancer, uterine corpus cancer, or soft tissue sarcoma: Multicenter, open-label phase 2 trial.Cancer Sci. 2019 Sep;110(9):2894-2904. doi: 10.1111/cas.14148. Epub 2019 Sep 3. Cancer Sci. 2019. PMID: 31348579 Free PMC article. Clinical Trial.
-
Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial.Lancet Oncol. 2015 Mar;16(3):257-65. doi: 10.1016/S1470-2045(15)70054-9. Epub 2015 Feb 20. Lancet Oncol. 2015. PMID: 25704439 Free PMC article. Clinical Trial.
-
Nivolumab: A Review in Advanced Melanoma.Drugs. 2015 Aug;75(12):1413-24. doi: 10.1007/s40265-015-0442-6. Drugs. 2015. PMID: 26220912 Review.
-
Temozolomide in uterine leiomyosarcomas.Gynecol Oncol. 2005 Jul;98(1):99-103. doi: 10.1016/j.ygyno.2005.03.018. Gynecol Oncol. 2005. PMID: 15916799 Review.
Cited by
-
Role of Immunotherapy in Sarcomas.Int J Mol Sci. 2024 Jan 19;25(2):1266. doi: 10.3390/ijms25021266. Int J Mol Sci. 2024. PMID: 38279265 Free PMC article. Review.
-
Exploring the landscape of immunotherapy approaches in sarcomas.Front Oncol. 2023 Jan 9;12:1069963. doi: 10.3389/fonc.2022.1069963. eCollection 2022. Front Oncol. 2023. PMID: 36686827 Free PMC article. Review.
-
Rationale and emerging strategies for immune checkpoint blockade in soft tissue sarcoma.Cancer. 2018 Oct 1;124(19):3819-3829. doi: 10.1002/cncr.31517. Epub 2018 May 3. Cancer. 2018. PMID: 29723407 Free PMC article. Review.
-
Combination immunotherapy with nivolumab and ipilimumab in patients with rare gynecological malignancies: results of the CA209-538 clinical trial.J Immunother Cancer. 2021 Nov;9(11):e003156. doi: 10.1136/jitc-2021-003156. J Immunother Cancer. 2021. PMID: 34782426 Free PMC article. Clinical Trial.
-
What Clinical Trials Are Needed for Treatment of Leiomyosarcoma?Curr Treat Options Oncol. 2022 Mar;23(3):439-449. doi: 10.1007/s11864-021-00928-y. Epub 2022 Mar 11. Curr Treat Options Oncol. 2022. PMID: 35275323 Free PMC article. Review.
References
-
- Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. - PubMed
-
- Zivanovic O, Leitao M, Lasonos A, Lindsay M, Jacks Q, Abu Rustum N. Stage-specific outcomes of patients with uterine leiomyosarcoma: a comparison of the International Federation of Gynecology and Obstetrics and American Joint Committee on Cancer Staging Systems. J Clin Oncol. 2009;27:2066–2072. - PMC - PubMed
-
- Echt G, Jepson J, Steel J, et al. Treatment of uterine sarcomas. Cancer. 1990;66:35–39. - PubMed
-
- Gadducci A, Landoni F, Sartori E, et al. Uterine leiomyosarcoma: analysis of treatment failures and survival. Gynecol Oncol. 1996;62:25–32. - PubMed
-
- Salazar OM, Bonfiglio TA, Patten SF, et al. Uterine sarcomas: natural history, treatment and prognosis. Cancer. 1978;42:1152–1160. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials