Evidence for Loss in Identity, De-Differentiation, and Trans-Differentiation of Islet β-Cells in Type 2 Diabetes
- PMID: 28424732
- PMCID: PMC5372778
- DOI: 10.3389/fgene.2017.00035
Evidence for Loss in Identity, De-Differentiation, and Trans-Differentiation of Islet β-Cells in Type 2 Diabetes
Abstract
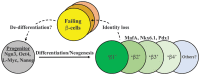
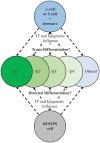
The two main types of diabetes mellitus have distinct etiologies, yet a similar outcome: loss of islet β-cell function that is solely responsible for the secretion of the insulin hormone to reduce elevated plasma glucose toward euglycemic levels. Type 1 diabetes (T1D) has traditionally been characterized by autoimmune-mediated β-cell death leading to insulin-dependence, whereas type 2 diabetes (T2D) has hallmarks of peripheral insulin resistance, β-cell dysfunction, and cell death. However, a growing body of evidence suggests that, especially during T2D, key components of β-cell failure involves: (1) loss of cell identity, specifically proteins associated with mature cell function (e.g., insulin and transcription factors like MAFA, PDX1, and NKX6.1), as well as (2) de-differentiation, defined by regression to a progenitor or stem cell-like state. New technologies have allowed the field to compare islet cell characteristics from normal human donors to those under pathophysiological conditions by single cell RNA-Sequencing and through epigenetic analysis. This has revealed a remarkable level of heterogeneity among histologically defined "insulin-positive" β-cells. These results not only suggest that these β-cell subsets have different responses to insulin secretagogues, but that defining their unique gene expression and epigenetic modification profiles will offer opportunities to develop cellular therapeutics to enrich/maintain certain subsets for correcting pathological glucose levels. In this review, we will summarize the recent literature describing how β-cell heterogeneity and plasticity may be influenced in T2D, and various possible avenues of therapeutic intervention.
Keywords: dedifferentiation; diabetes mellitus; epigenetics; islets of Langerhans; trans-differentiation; transcription factors; type 2; β-cell.
Figures
Similar articles
-
Pancreatic β-cell identity, glucose sensing and the control of insulin secretion.Biochem J. 2015 Mar 1;466(2):203-18. doi: 10.1042/BJ20141384. Biochem J. 2015. PMID: 25697093 Review.
-
Alterations in Beta Cell Identity in Type 1 and Type 2 Diabetes.Curr Diab Rep. 2019 Aug 10;19(9):83. doi: 10.1007/s11892-019-1194-6. Curr Diab Rep. 2019. PMID: 31401713 Free PMC article. Review.
-
Influence of diabetes on the loss of beta cell differentiation after islet transplantation in rats.Diabetologia. 2007 Oct;50(10):2117-25. doi: 10.1007/s00125-007-0749-2. Epub 2007 Jul 20. Diabetologia. 2007. PMID: 17641871
-
Whole-Genome Bisulfite Sequencing of Human Pancreatic Islets Reveals Novel Differentially Methylated Regions in Type 2 Diabetes Pathogenesis.Diabetes. 2017 Apr;66(4):1074-1085. doi: 10.2337/db16-0996. Epub 2017 Jan 4. Diabetes. 2017. PMID: 28052964
-
Banting lecture 1990. Beta-cells in type II diabetes mellitus.Diabetes. 1991 Feb;40(2):166-80. doi: 10.2337/diab.40.2.166. Diabetes. 1991. PMID: 1991568 Review.
Cited by
-
Hierarchical progressive learning of cell identities in single-cell data.Nat Commun. 2021 May 14;12(1):2799. doi: 10.1038/s41467-021-23196-8. Nat Commun. 2021. PMID: 33990598 Free PMC article.
-
Adar1 deletion causes degeneration of the exocrine pancreas via Mavs-dependent interferon signaling.Development. 2023 Jan 15;150(2):dev201097. doi: 10.1242/dev.201097. Epub 2023 Jan 16. Development. 2023. PMID: 36458554 Free PMC article.
-
Polycomb Repressive Complexes: Shaping Pancreatic Beta-Cell Destiny in Development and Metabolic Disease.Front Cell Dev Biol. 2022 May 4;10:868592. doi: 10.3389/fcell.2022.868592. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35602600 Free PMC article. Review.
-
Lipid Droplets Protect Human β-Cells From Lipotoxicity-Induced Stress and Cell Identity Changes.Diabetes. 2021 Nov;70(11):2595-2607. doi: 10.2337/db21-0261. Epub 2021 Aug 25. Diabetes. 2021. PMID: 34433630 Free PMC article.
-
Ghrelin deletion and conditional ghrelin cell ablation increase pancreatic islet size in mice.J Clin Invest. 2023 Dec 15;133(24):e169349. doi: 10.1172/JCI169349. J Clin Invest. 2023. PMID: 38099492 Free PMC article.
References
-
- Arystarkhova E., Liu Y. B., Salazar C., Stanojevic V., Clifford R. J., Kaplan J. H., et al. . (2013). Hyperplasia of pancreatic beta cells and improved glucose tolerance in mice deficient in the FXYD2 subunit of Na,K-ATPase. J. Biol. Chem. 288, 7077–7085. 10.1074/jbc.M112.401190 - DOI - PMC - PubMed
-
- Bernard-Kargar C., Kassis N., Berthault M. F., Pralong W., Ktorza A. (2001). Sialylated form of the neural cell adhesion molecule (NCAM): a new tool for the identification and sorting of beta-cell subpopulations with different functional activity. Diabetes 50(Suppl. 1), S125–S130. 10.2337/diabetes.50.2007.s125 - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

