Mechanisms of corticosteroid insensitivity in COPD alveolar macrophages exposed to NTHi
- PMID: 28420398
- PMCID: PMC5395788
- DOI: 10.1186/s12931-017-0539-4
Mechanisms of corticosteroid insensitivity in COPD alveolar macrophages exposed to NTHi
Abstract
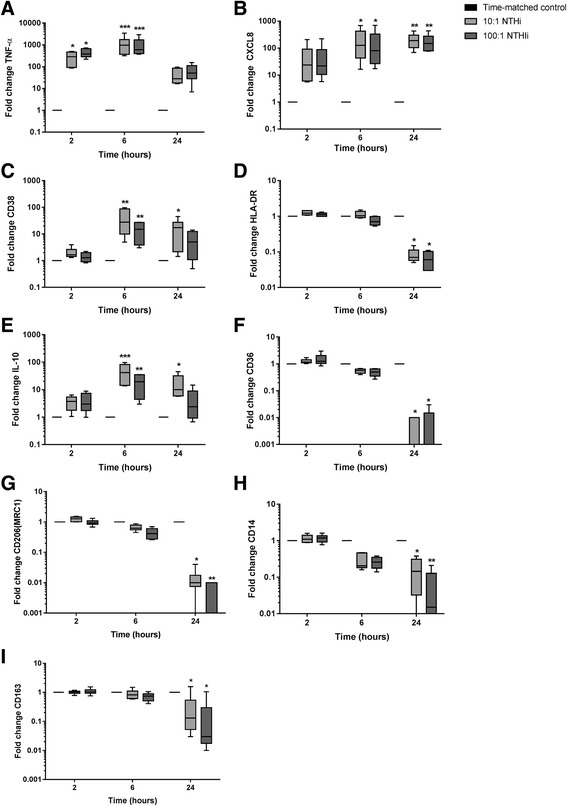
Background: Non-typeable Haemophilus influenza (NTHi) infection is common in COPD. Corticosteroids can have limited therapeutic effects in COPD patients. NTHi causes corticosteroid insensitive cytokine production from COPD alveolar macrophages. We investigated the mechanisms by which NTHi causes corticosteroid insensitive inflammatory responses, and the effects of NTHi exposure on COPD macrophage polarisation.
Method: Alveolar macrophages from COPD patients and controls were exposed to NTHi in conjunction with the corticosteroid dexamethasone and/or the p38 MAPK inhibitor BIRB-796. Cytokine release, GR phosphorylation and modulation and macrophage phenotype were analysed.
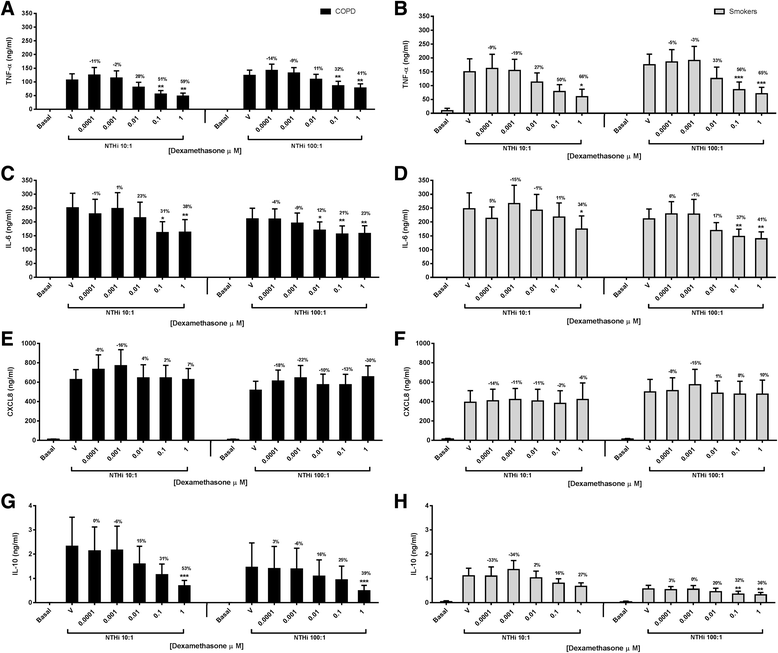
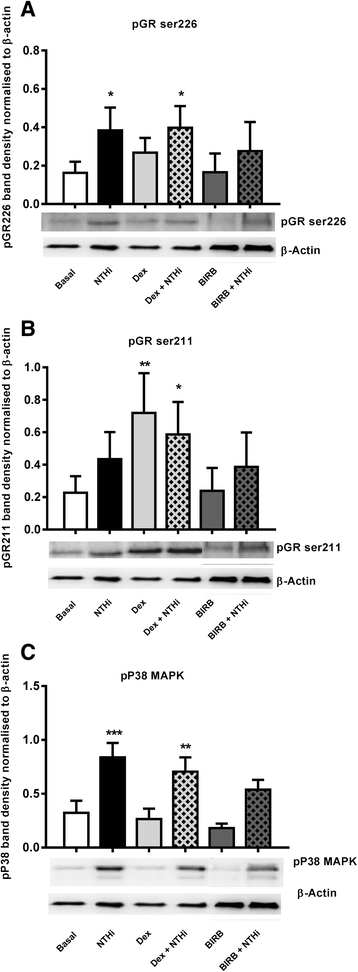
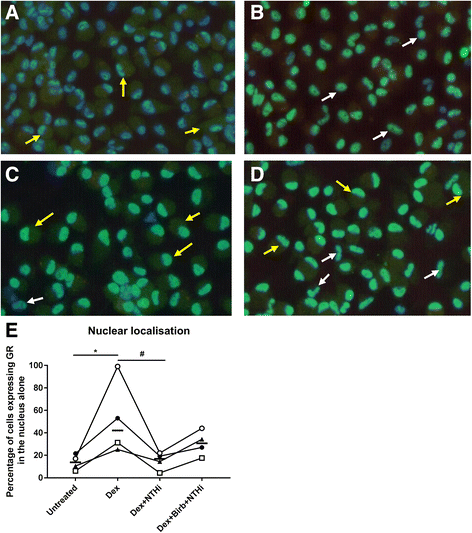
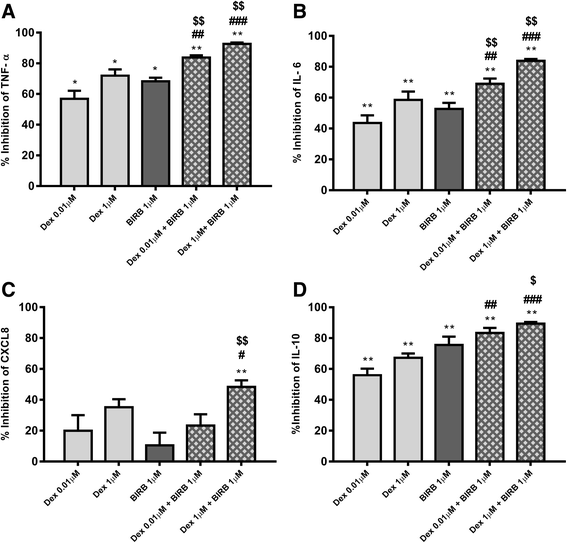
Results: Dexamethasone significantly inhibited NTHi induced TNF-α, IL-6 and IL-10 from COPD macrophages but, CXCL8 was not suppressed. BIRB-796 combined with dexamethasone caused significantly greater inhibition of all cytokines than either drug alone (p < 0.05 all comparisons). NTHi caused phosphorylation of GR S226 reducing GR nuclear localisation, an effect regulated by p38 MAPK. NTHi altered macrophage polarisation by increasing IL-10 and decreasing CD36, CD206, CD163 and HLA-DR.
Conclusion: NTHi exposure causes p38 MAPK dependent GR phosphorylation associated with decreased GR function in COPD alveolar macrophages. Combining a p38 MAPK inhibitor with corticosteroids can enhance anti-inflammatory effects during NTHi exposure of COPD alveolar macrophages. NTHi causes macrophage polarisation that favours bacterial persistence.
Figures
Similar articles
-
Synergistic effects of p38 mitogen-activated protein kinase inhibition with a corticosteroid in alveolar macrophages from patients with chronic obstructive pulmonary disease.J Pharmacol Exp Ther. 2011 Sep;338(3):732-40. doi: 10.1124/jpet.111.180737. Epub 2011 May 24. J Pharmacol Exp Ther. 2011. PMID: 21610141
-
Corticosteroid insensitive alveolar macrophages from asthma patients; synergistic interaction with a p38 mitogen-activated protein kinase (MAPK) inhibitor.Br J Clin Pharmacol. 2015 May;79(5):756-66. doi: 10.1111/bcp.12536. Br J Clin Pharmacol. 2015. PMID: 25358442 Free PMC article.
-
Reversal of corticosteroid insensitivity by p38 MAPK inhibition in peripheral blood mononuclear cells from COPD.Int J Chron Obstruct Pulmon Dis. 2015 Feb 4;10:283-91. doi: 10.2147/COPD.S72403. eCollection 2015. Int J Chron Obstruct Pulmon Dis. 2015. PMID: 25678784 Free PMC article.
-
The Interplay Between Immune Response and Bacterial Infection in COPD: Focus Upon Non-typeable Haemophilus influenzae.Front Immunol. 2018 Nov 5;9:2530. doi: 10.3389/fimmu.2018.02530. eCollection 2018. Front Immunol. 2018. PMID: 30455693 Free PMC article. Review.
-
Nontypeable Haemophilus influenzae and chronic obstructive pulmonary disease: a review for clinicians.Crit Rev Microbiol. 2018 Mar;44(2):125-142. doi: 10.1080/1040841X.2017.1329274. Epub 2017 May 25. Crit Rev Microbiol. 2018. PMID: 28539074 Review.
Cited by
-
OR2AT4 and OR1A2 counterregulate molecular pathophysiological processes of steroid-resistant inflammatory lung diseases in human alveolar macrophages.Mol Med. 2022 Dec 12;28(1):150. doi: 10.1186/s10020-022-00572-8. Mol Med. 2022. PMID: 36503361 Free PMC article.
-
How inhaled corticosteroids target inflammation in COPD.Eur Respir Rev. 2023 Oct 18;32(170):230084. doi: 10.1183/16000617.0084-2023. Print 2023 Dec 31. Eur Respir Rev. 2023. PMID: 37852657 Free PMC article. Review.
-
Assessment of bacterial exposure on phagocytic capability and surface marker expression of sputum macrophages and neutrophils in COPD patients.Clin Exp Immunol. 2021 Oct;206(1):99-109. doi: 10.1111/cei.13638. Epub 2021 Jul 12. Clin Exp Immunol. 2021. PMID: 34143447 Free PMC article. Clinical Trial.
-
Differential responses of COPD macrophages to respiratory bacterial pathogens.ERJ Open Res. 2022 Aug 1;8(3):00044-2022. doi: 10.1183/23120541.00044-2022. eCollection 2022 Jul. ERJ Open Res. 2022. PMID: 35923420 Free PMC article.
-
Effects of Inhaled Corticosteroids on the Innate Immunological Response to Pseudomonas aeruginosa Infection in Patients with COPD.Int J Mol Sci. 2022 Jul 23;23(15):8127. doi: 10.3390/ijms23158127. Int J Mol Sci. 2022. PMID: 35897707 Free PMC article.
References
-
- GOLD guidelines. [www.goldcopd.org]. Accessed Sept 2014.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

