A TSPO ligand attenuates brain injury after intracerebral hemorrhage
- PMID: 28416580
- PMCID: PMC5503714
- DOI: 10.1096/fj.201601377RR
A TSPO ligand attenuates brain injury after intracerebral hemorrhage
Abstract
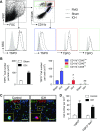
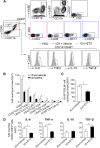
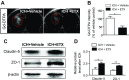
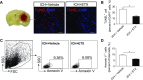
Intracerebral hemorrhage (ICH) is a devastating disease without effective treatment. After ICH, the immediate infiltration of leukocytes and activation of microglia are accompanied by a rapid up-regulation of the 18-kDa translocator protein (TSPO). TSPO ligands have shown anti-inflammatory and neuroprotective properties in models of CNS injury. In this study, we determined the impact of a TSPO ligand, etifoxine, on brain injury and inflammation in 2 mouse models of ICH. TSPO was up-regulated in Iba1+ cells from brains of patients with ICH and in CD11b+CD45int cells from mice subjected to collagenase-induced ICH. Etifoxine significantly reduced neurodeficits and perihematomal brain edema after ICH induction by injection of either autologous blood or collagenase. In collagenase-induced ICH mice, the protection of etifoxine was associated with reduced leukocyte infiltration into the brain and microglial production of IL-6 and TNF-α. Etifoxine improved blood-brain barrier integrity and diminished cell death. Notably, the protective effect of etifoxine was abolished in mice depleted of microglia by using a colony-stimulating factor 1 receptor inhibitor. These results indicate that the TSPO ligand etifoxine attenuates brain injury and inflammation after ICH. TSPO may be a viable therapeutic target that requires further investigations in ICH.-Li, M., Ren, H., Sheth, K. N., Shi, F.-D., Liu, Q. A TSPO ligand attenuates brain injury after intracerebral hemorrhage.
Keywords: etifoxine; hemorrhagic stroke; immune modulation; inflammation.
© The Author(s).
Figures

Similar articles
-
A translocator protein 18 kDa agonist protects against cerebral ischemia/reperfusion injury.J Neuroinflammation. 2017 Jul 28;14(1):151. doi: 10.1186/s12974-017-0921-7. J Neuroinflammation. 2017. PMID: 28754131 Free PMC article.
-
Selective NLRP3 (Pyrin Domain-Containing Protein 3) Inflammasome Inhibitor Reduces Brain Injury After Intracerebral Hemorrhage.Stroke. 2018 Jan;49(1):184-192. doi: 10.1161/STROKEAHA.117.018904. Epub 2017 Dec 6. Stroke. 2018. PMID: 29212744 Free PMC article.
-
Augmented expression of TSPO after intracerebral hemorrhage: a role in inflammation?J Neuroinflammation. 2016 Jun 17;13(1):151. doi: 10.1186/s12974-016-0619-2. J Neuroinflammation. 2016. PMID: 27315802 Free PMC article.
-
Axonal regeneration and neuroinflammation: roles for the translocator protein 18 kDa.J Neuroendocrinol. 2012 Jan;24(1):71-81. doi: 10.1111/j.1365-2826.2011.02215.x. J Neuroendocrinol. 2012. PMID: 21951109 Review.
-
Diagnostic and Therapeutic Potential of TSPO Studies Regarding Neurodegenerative Diseases, Psychiatric Disorders, Alcohol Use Disorders, Traumatic Brain Injury, and Stroke: An Update.Cells. 2020 Apr 2;9(4):870. doi: 10.3390/cells9040870. Cells. 2020. PMID: 32252470 Free PMC article. Review.
Cited by
-
Neurosteroid Metabolites of Gonadal Steroid Hormones in Neuroprotection: Implications for Sex Differences in Neurodegenerative Disease.Front Mol Neurosci. 2018 Oct 5;11:359. doi: 10.3389/fnmol.2018.00359. eCollection 2018. Front Mol Neurosci. 2018. PMID: 30344476 Free PMC article. Review.
-
Dexmedetomidine Alleviates Intracerebral Hemorrhage-Induced Anxiety-Like Behaviors in Mice Through the Inhibition of TRPV4 Opening.Front Pharmacol. 2022 Apr 1;13:852401. doi: 10.3389/fphar.2022.852401. eCollection 2022. Front Pharmacol. 2022. PMID: 35431940 Free PMC article.
-
CCL5 mediated astrocyte-T cell interaction disrupts blood-brain barrier in mice after hemorrhagic stroke.J Cereb Blood Flow Metab. 2024 Mar;44(3):367-383. doi: 10.1177/0271678X231214838. Epub 2023 Nov 16. J Cereb Blood Flow Metab. 2024. PMID: 37974301 Free PMC article.
-
Translocator protein (18 kDa) regulates the microglial phenotype in Parkinson's disease through P47.Bioengineered. 2022 Apr;13(4):11061-11071. doi: 10.1080/21655979.2022.2068754. Bioengineered. 2022. PMID: 35475466 Free PMC article.
-
Intranasal Delivery of Gene-Edited Microglial Exosomes Improves Neurological Outcomes after Intracerebral Hemorrhage by Regulating Neuroinflammation.Brain Sci. 2023 Apr 8;13(4):639. doi: 10.3390/brainsci13040639. Brain Sci. 2023. PMID: 37190604 Free PMC article.
References
-
- Van Asch C. J., Luitse M. J., Rinkel G. J., van der Tweel I., Algra A., Klijn C. J. (2010) Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol. 9, 167–176 - PubMed
-
- INTERACT2 Investigators (2015) Mannitol and outcome in intracerebral hemorrhage: propensity score and multivariable intensive blood pressure reduction in acute cerebral hemorrhage trial 2 results. Stroke 46, 2762–2767 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

