A phase 1 study of the CXCR4 antagonist plerixafor in combination with high-dose cytarabine and etoposide in children with relapsed or refractory acute leukemias or myelodysplastic syndrome: A Pediatric Oncology Experimental Therapeutics Investigators' Consortium study (POE 10-03)
- PMID: 28409853
- PMCID: PMC5675008
- DOI: 10.1002/pbc.26414
A phase 1 study of the CXCR4 antagonist plerixafor in combination with high-dose cytarabine and etoposide in children with relapsed or refractory acute leukemias or myelodysplastic syndrome: A Pediatric Oncology Experimental Therapeutics Investigators' Consortium study (POE 10-03)
Abstract
Background: Plerixafor, a reversible CXCR4 antagonist, inhibits interactions between leukemic blasts and the bone marrow stromal microenvironment and may enhance chemosensitivity. A phase 1 trial of plerixafor in combination with intensive chemotherapy in children and young adults with relapsed or refractory acute lymphoblastic leukemia (ALL), acute myeloid leukemia (AML), and myelodysplastic syndrome (MDS) was performed to determine a tolerable and biologically active dose.
Procedure: Plerixafor was administered daily for 5 days at four dose levels (6, 9, 12, and 15 mg/m2 /dose) followed 4 hr later by high-dose cytarabine (every 12 hr) and etoposide (daily).
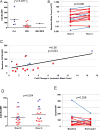
Results: Nineteen patients (13 with AML, 5 with ALL, 1 with MDS) were treated. The most common grade 3 or greater nonhematologic toxicities attributable to plerixafor were febrile neutropenia and hypokalemia. There were no dose-limiting toxicities (DLTs). Plerixafor exposure increased with increasing dose levels and clearance was similar on days 1 and 5. Eighteen patients were evaluable for response. Two patients achieved complete remission (CR) and one patient achieved CR with incomplete hematologic recovery (CRi): all three had AML. No responses were seen in patients with ALL or MDS. Plerixafor mobilized leukemic blasts into the peripheral blood in 14 of 16 evaluable patients (median 3.4-fold increase), and the degree of mobilization correlated with surface CXCR4 expression.
Conclusions: Plerixafor, in combination with high-dose cytarabine and etoposide, was well tolerated in children and young adults with relapsed/refractory acute leukemias and MDS. While biologic responses were observed, clinical responses in this heavily pretreated cohort were modest.
Keywords: CXCR4; leukemia; pediatric; plerixafor; tumor microenvironment.
© 2017 Wiley Periodicals, Inc.
Conflict of interest statement
The authors have no relevant financial interests to disclose.
Figures
Similar articles
-
A phase 1/2 study of chemosensitization with the CXCR4 antagonist plerixafor in relapsed or refractory acute myeloid leukemia.Blood. 2012 Apr 26;119(17):3917-24. doi: 10.1182/blood-2011-10-383406. Epub 2012 Feb 2. Blood. 2012. PMID: 22308295 Free PMC article. Clinical Trial.
-
Twice-daily fludarabine and cytarabine combination with or without gentuzumab ozogamicin is effective in patients with relapsed/refractory acute myeloid leukemia, high-risk myelodysplastic syndrome, and blast- phase chronic myeloid leukemia.Clin Lymphoma Myeloma Leuk. 2012 Aug;12(4):244-51. doi: 10.1016/j.clml.2012.03.003. Epub 2012 Apr 24. Clin Lymphoma Myeloma Leuk. 2012. PMID: 22534616 Free PMC article. Clinical Trial.
-
A phase I-II study of plerixafor in combination with fludarabine, idarubicin, cytarabine, and G-CSF (PLERIFLAG regimen) for the treatment of patients with the first early-relapsed or refractory acute myeloid leukemia.Ann Hematol. 2018 May;97(5):763-772. doi: 10.1007/s00277-018-3229-5. Epub 2018 Feb 2. Ann Hematol. 2018. PMID: 29392425 Clinical Trial.
-
Therapy-related myelodysplastic syndrome with monosomy 5 after successful treatment of acute myeloid leukemia (M2).Am J Hematol. 2005 Jun;79(2):136-41. doi: 10.1002/ajh.20329. Am J Hematol. 2005. PMID: 15929101 Review.
-
Etoposide in acute leukemia. Past experience and future perspectives.Cancer. 1991 Jan 1;67(1 Suppl):281-4. doi: 10.1002/1097-0142(19910101)67:1+<281::aid-cncr2820671312>3.0.co;2-h. Cancer. 1991. PMID: 1984828 Review.
Cited by
-
Support of acute lymphoblastic leukemia cells by nonmalignant bone marrow stromal cells.Oncol Lett. 2019 Jun;17(6):5039-5049. doi: 10.3892/ol.2019.10188. Epub 2019 Mar 22. Oncol Lett. 2019. PMID: 31186715 Free PMC article.
-
The Current Status and Future Potential of Theranostics to Diagnose and Treat Childhood Cancer.Front Oncol. 2020 Nov 19;10:578286. doi: 10.3389/fonc.2020.578286. eCollection 2020. Front Oncol. 2020. PMID: 33330054 Free PMC article. Review.
-
The Role of CXCR1, CXCR2, CXCR3, CXCR5, and CXCR6 Ligands in Molecular Cancer Processes and Clinical Aspects of Acute Myeloid Leukemia (AML).Cancers (Basel). 2023 Sep 14;15(18):4555. doi: 10.3390/cancers15184555. Cancers (Basel). 2023. PMID: 37760523 Free PMC article. Review.
-
Cancer stem cells: a major culprit of intra-tumor heterogeneity.Am J Cancer Res. 2021 Dec 15;11(12):5782-5811. eCollection 2021. Am J Cancer Res. 2021. PMID: 35018226 Free PMC article. Review.
-
Clinical Aspects and Significance of β-Chemokines, γ-Chemokines, and δ-Chemokines in Molecular Cancer Processes in Acute Myeloid Leukemia (AML) and Myelodysplastic Neoplasms (MDS).Cancers (Basel). 2024 Sep 24;16(19):3246. doi: 10.3390/cancers16193246. Cancers (Basel). 2024. PMID: 39409868 Free PMC article. Review.
References
-
- Hunger SP, Raetz EA, Loh ML, Mullighan CG. Improving outcomes for high-risk ALL: translating new discoveries into clinical care. Pediatr Blood Cancer. 2011;56:984–993. - PubMed
-
- Manabe A, Coustan-Smith E, Behm FG, Raimondi SC, Campana D. Bone marrow-derived stromal cells prevent apoptotic cell death in B-lineage acute lymphoblastic leukemia. Blood. 1992;79:2370–2377. - PubMed
-
- Bendall LJ, Daniel A, Kortlepel K, Gottlieb DJ. Bone marrow adherent layers inhibit apoptosis of acute myeloid leukemia cells. Exp Hematol. 1994;22:1252–1260. - PubMed
-
- Burger JA, Tsukada N, Burger M, Zvaifler NJ, Dell'Aquila M, Kipps TJ. Blood-derived nurse-like cells protect chronic lymphocytic leukemia B cells from spontaneous apoptosis through stromal cell-derived factor-1. Blood. 2000;96:2655–2663. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

