Drug Induced Pneumonitis Secondary to Treatment with Paritaprevir/Ritonavir/Ombitasvir and Dasabuvir (VIEKIRA PAK®) for Chronic Hepatitis C: Case Report of an Unexpected Life-Threatening Adverse Reaction
- PMID: 28408931
- PMCID: PMC5376942
- DOI: 10.1155/2017/4895736
Drug Induced Pneumonitis Secondary to Treatment with Paritaprevir/Ritonavir/Ombitasvir and Dasabuvir (VIEKIRA PAK®) for Chronic Hepatitis C: Case Report of an Unexpected Life-Threatening Adverse Reaction
Abstract
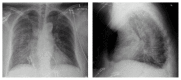
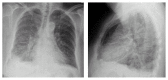
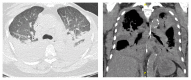
VIEKIRA PAK (ritonavir-boosted paritaprevir/ombitasvir and dasabuvir) is an approved treatment for compensated patients with genotype 1 (GT1) chronic hepatitis C virus (HCV) infection. This oral regimen has minimal adverse effects and is well tolerated. Cure rates are 97% in patients infected with HCV GT 1a and 99% in those with HCV GT 1b. We report the first case of life-threatening allergic pneumonitis associated with VIEKIRA PAK. This unexpected serious adverse event occurred in a 68-year-old Chinese female with genotype 1b chronic hepatitis C and Child-Pugh A cirrhosis. One week into treatment with VIEKIRA PAK without ribavirin, she was admitted to hospital with respiratory distress and acute kidney injury requiring intensive care input. She was initially diagnosed with community acquired pneumonia and improved promptly with intravenous antibiotics and supported care. No bacterial or viral pathogens were cultured. Following complete recovery, she recommenced VIEKIRA PAK but represented 5 days later with more rapidly progressive respiratory failure, requiring intubation and ventilation, inotropic support, and haemodialysis. The final diagnosis was drug induced pneumonitis.
Figures
Similar articles
-
New combination antiviral for the treatment of hepatitis C.Am J Health Syst Pharm. 2016 Jul 15;73(14):1042-50. doi: 10.2146/ajhp150163. Epub 2016 May 23. Am J Health Syst Pharm. 2016. PMID: 27217519 Review.
-
Paritaprevir/ritonavir/ombitasvir plus dasabuvir with ribavirin for chronic hepatitis C.Aust Prescr. 2016 Aug;39(4):141-143. doi: 10.18773/austprescr.2016.045. Epub 2016 May 20. Aust Prescr. 2016. PMID: 27756980 Free PMC article. No abstract available.
-
Population Pharmacokinetics of Paritaprevir, Ombitasvir, Dasabuvir, Ritonavir, and Ribavirin in Hepatitis C Virus-Infected Cirrhotic and Non-cirrhotic Patients: Analyses Across Nine Phase III Studies.Clin Pharmacokinet. 2018 Nov;57(11):1407-1419. doi: 10.1007/s40262-018-0640-y. Clin Pharmacokinet. 2018. PMID: 29516428
-
Viekira Pak (Ombitasvir, Paritaprevir, and Ritonavir Tablets; Dasabuvir Tablets): All-Oral Fixed Combination Approved for Genotype 1 Chronic Hepatitis C Infection.Am Health Drug Benefits. 2015 Mar;8(Spec Feature):142-7. Am Health Drug Benefits. 2015. PMID: 26629280 Free PMC article. No abstract available.
-
Real-world experience with ombitasvir/paritaprevir boosted with ritonavir and possibly combined with dasabuvir and ribavirin in HCV infection.Clin Exp Hepatol. 2016 Jun;2(2):34-37. doi: 10.5114/ceh.2016.60247. Epub 2016 Jun 6. Clin Exp Hepatol. 2016. PMID: 28856270 Free PMC article. Review.
Cited by
-
Ombitasvir/paritaprevir/ritonavir+dasabuvir and ribavirin associated drug-induced liver injury and syndrome of inappropriate secretion of anti-diuretic hormone: A case report.Clin Mol Hepatol. 2019 Sep;25(3):326-330. doi: 10.3350/cmh.2018.0063. Epub 2018 Dec 4. Clin Mol Hepatol. 2019. PMID: 30509013 Free PMC article. No abstract available.
-
VIEKIRA PAK associated drug-induced interstitial lung disease: Case series with systematic review of literature.Clin Mol Hepatol. 2019 Jun;25(2):218-222. doi: 10.3350/cmh.2018.0017. Epub 2018 Sep 6. Clin Mol Hepatol. 2019. PMID: 30184617 Free PMC article. No abstract available.
References
-
- World Health Organisation. Hepatitis C. http://www.who.int/mediacentre/factsheets/fs164/en/
-
- Gane E., Stedman C., Brunton C., et al. Impact of improved treatment on disease burden of chronic hepatitis C in New Zealand. New Zealand Medical Journal. 2014;127(1407):61–74. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous