Age-Associated Microbial Dysbiosis Promotes Intestinal Permeability, Systemic Inflammation, and Macrophage Dysfunction
- PMID: 28407483
- PMCID: PMC5392495
- DOI: 10.1016/j.chom.2017.03.002
Age-Associated Microbial Dysbiosis Promotes Intestinal Permeability, Systemic Inflammation, and Macrophage Dysfunction
Erratum in
-
Age-Associated Microbial Dysbiosis Promotes Intestinal Permeability, Systemic Inflammation, and Macrophage Dysfunction.Cell Host Microbe. 2018 Apr 11;23(4):570. doi: 10.1016/j.chom.2018.03.006. Cell Host Microbe. 2018. PMID: 29649447 Free PMC article. No abstract available.
Abstract
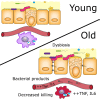
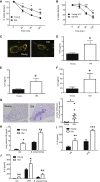
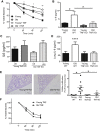
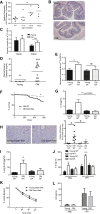
Levels of inflammatory mediators in circulation are known to increase with age, but the underlying cause of this age-associated inflammation is debated. We find that, when maintained under germ-free conditions, mice do not display an age-related increase in circulating pro-inflammatory cytokine levels. A higher proportion of germ-free mice live to 600 days than their conventional counterparts, and macrophages derived from aged germ-free mice maintain anti-microbial activity. Co-housing germ-free mice with old, but not young, conventionally raised mice increases pro-inflammatory cytokines in the blood. In tumor necrosis factor (TNF)-deficient mice, which are protected from age-associated inflammation, age-related microbiota changes are not observed. Furthermore, age-associated microbiota changes can be reversed by reducing TNF using anti-TNF therapy. These data suggest that aging-associated microbiota promote inflammation and that reversing these age-related microbiota changes represents a potential strategy for reducing age-associated inflammation and the accompanying morbidity.
Keywords: Streptococcus pneumoniae; elderly; host defense; immunosenescence; inflamm-aging; inflammation; macrophage; microbiome; microbiota.
Copyright © 2017 The Authors. Published by Elsevier Inc. All rights reserved.
Figures

Comment on
-
Breaking Down Walls: Microbiota and the Aging Gut.Cell Host Microbe. 2017 Apr 12;21(4):417-418. doi: 10.1016/j.chom.2017.03.013. Cell Host Microbe. 2017. PMID: 28407478
Similar articles
-
Dysbiotic gut microbiota causes transmissible Crohn's disease-like ileitis independent of failure in antimicrobial defence.Gut. 2016 Feb;65(2):225-37. doi: 10.1136/gutjnl-2015-309333. Epub 2015 Apr 17. Gut. 2016. PMID: 25887379 Free PMC article.
-
A Polysaccharide Isolated from Dictyophora indusiata Promotes Recovery from Antibiotic-Driven Intestinal Dysbiosis and Improves Gut Epithelial Barrier Function in a Mouse Model.Nutrients. 2018 Jul 31;10(8):1003. doi: 10.3390/nu10081003. Nutrients. 2018. PMID: 30065236 Free PMC article.
-
The Gut Microbiota and Unhealthy Aging: Disentangling Cause from Consequence.Cell Host Microbe. 2020 Aug 12;28(2):180-189. doi: 10.1016/j.chom.2020.07.013. Cell Host Microbe. 2020. PMID: 32791111 Review.
-
Dysbiosis-induced intestinal inflammation activates tumor necrosis factor receptor I and mediates alcoholic liver disease in mice.Hepatology. 2015 Mar;61(3):883-94. doi: 10.1002/hep.27489. Epub 2015 Jan 30. Hepatology. 2015. PMID: 25251280 Free PMC article.
-
Connecting the immune system, systemic chronic inflammation and the gut microbiome: The role of sex.J Autoimmun. 2018 Aug;92:12-34. doi: 10.1016/j.jaut.2018.05.008. Epub 2018 Jun 1. J Autoimmun. 2018. PMID: 29861127 Review.
Cited by
-
Probing the Interface of HIV and Inflammaging.Curr HIV/AIDS Rep. 2021 Jun;18(3):198-210. doi: 10.1007/s11904-021-00547-0. Epub 2021 Mar 11. Curr HIV/AIDS Rep. 2021. PMID: 33709322 Review.
-
Neuronal induction of BNIP3-mediated mitophagy slows systemic aging in Drosophila.Nat Aging. 2022 Jun;2(6):494-507. doi: 10.1038/s43587-022-00214-y. Epub 2022 May 16. Nat Aging. 2022. PMID: 36213625 Free PMC article.
-
Gut Microbiota Interaction with the Central Nervous System throughout Life.J Clin Med. 2021 Mar 21;10(6):1299. doi: 10.3390/jcm10061299. J Clin Med. 2021. PMID: 33801153 Free PMC article. Review.
-
Early Life Exposure to Food Contaminants and Social Stress as Risk Factor for Metabolic Disorders Occurrence?-An Overview.Biomolecules. 2021 May 3;11(5):687. doi: 10.3390/biom11050687. Biomolecules. 2021. PMID: 34063694 Free PMC article. Review.
-
Immunosenescence, Inflammaging, and Lung Senescence in Asthma in the Elderly.Biomolecules. 2022 Oct 11;12(10):1456. doi: 10.3390/biom12101456. Biomolecules. 2022. PMID: 36291665 Free PMC article. Review.
References
-
- Antunes G., Evans S.A., Lordan J.L., Frew A.J. Systemic cytokine levels in community-acquired pneumonia and their association with disease severity. Eur. Respir. J. 2002;20:990–995. - PubMed
-
- Bartosch S., Fite A., Macfarlane G.T., McMurdo M.E. Characterization of bacterial communities in feces from healthy elderly volunteers and hospitalized elderly patients by using real-time PCR and effects of antibiotic treatment on the fecal microbiota. Appl. Environ. Microbiol. 2004;70:3575–3581. - PMC - PubMed
-
- Brenchley J.M., Price D.A., Schacker T.W., Asher T.E., Silvestri G., Rao S., Kazzaz Z., Bornstein E., Lambotte O., Altmann D. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat. Med. 2006;12:1365–1371. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

