miR-424(322)/503 is a breast cancer tumor suppressor whose loss promotes resistance to chemotherapy
- PMID: 28404630
- PMCID: PMC5393051
- DOI: 10.1101/gad.292318.116
miR-424(322)/503 is a breast cancer tumor suppressor whose loss promotes resistance to chemotherapy
Abstract
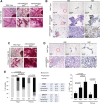
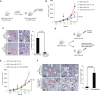
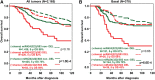
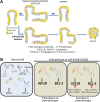
The female mammary gland is a very dynamic organ that undergoes continuous tissue remodeling during adulthood. Although it is well established that the number of menstrual cycles and pregnancy (in this case transiently) increase the risk of breast cancer, the reasons are unclear. Growing clinical and experimental evidence indicates that improper involution plays a role in the development of this malignancy. Recently, we described the miR-424(322)/503 cluster as an important regulator of mammary epithelial involution after pregnancy. Here, through the analysis of ∼3000 primary tumors, we show that miR-424(322)/503 is commonly lost in a subset of aggressive breast cancers and describe the genetic aberrations that inactivate its expression. Furthermore, through the use of a knockout mouse model, we demonstrate for the first time that loss of miR-424(322)/503 promotes breast tumorigenesis in vivo. Remarkably, we found that loss of miR-424(322)/503 promotes chemoresistance due to the up-regulation of two of its targets: BCL-2 and insulin-like growth factor-1 receptor (IGF1R). Importantly, targeted therapies blocking the aberrant activity of these targets restore sensitivity to chemotherapy. Overall, our studies reveal miR-424(322)/503 as a tumor suppressor in breast cancer and provide a link between mammary epithelial involution, tumorigenesis, and the phenomenon of chemoresistance.
Keywords: breast cancer; chemoresistance; microRNA; tumor suppressor.
© 2017 Rodriguez-Barrueco et al.; Published by Cold Spring Harbor Laboratory Press.
Figures


Similar articles
-
The miR-424(322)/503 cluster orchestrates remodeling of the epithelium in the involuting mammary gland.Genes Dev. 2014 Apr 1;28(7):765-82. doi: 10.1101/gad.237404.114. Epub 2014 Mar 17. Genes Dev. 2014. PMID: 24636986 Free PMC article.
-
Tumor-promoting properties of miR-8084 in breast cancer through enhancing proliferation, suppressing apoptosis and inducing epithelial-mesenchymal transition.J Transl Med. 2018 Feb 23;16(1):38. doi: 10.1186/s12967-018-1419-5. J Transl Med. 2018. PMID: 29471858 Free PMC article.
-
MicroRNA-148a promotes apoptosis and suppresses growth of breast cancer cells by targeting B-cell lymphoma 2.Anticancer Drugs. 2017 Jul;28(6):588-595. doi: 10.1097/CAD.0000000000000498. Anticancer Drugs. 2017. PMID: 28430743
-
Bcl-2 gene family and related proteins in mammary gland involution and breast cancer.J Mammary Gland Biol Neoplasia. 1999 Apr;4(2):153-64. doi: 10.1023/a:1018773123899. J Mammary Gland Biol Neoplasia. 1999. PMID: 10426394 Review.
-
The Role of MicroRNAs in the Chemoresistance of Breast Cancer.Drug Dev Res. 2015 Nov;76(7):368-74. doi: 10.1002/ddr.21275. Epub 2015 Aug 27. Drug Dev Res. 2015. PMID: 26310899 Review.
Cited by
-
Potential molecular mechanism in self-renewal is associated with miRNA dysregulation in sacral chordoma - A next-generation RNA sequencing study.Heliyon. 2022 Aug 13;8(8):e10227. doi: 10.1016/j.heliyon.2022.e10227. eCollection 2022 Aug. Heliyon. 2022. PMID: 36033338 Free PMC article.
-
MicroRNA-dependent inhibition of WEE1 controls cancer stem-like characteristics and malignant behavior in ovarian cancer.Mol Ther Nucleic Acids. 2022 Aug 24;29:803-822. doi: 10.1016/j.omtn.2022.08.028. eCollection 2022 Sep 13. Mol Ther Nucleic Acids. 2022. PMID: 36159587 Free PMC article.
-
A novel miRNA identified in GRSF1 complex drives the metastasis via the PIK3R3/AKT/NF-κB and TIMP3/MMP9 pathways in cervical cancer cells.Cell Death Dis. 2019 Sep 2;10(9):636. doi: 10.1038/s41419-019-1841-5. Cell Death Dis. 2019. PMID: 31474757 Free PMC article.
-
A two-microRNA signature as a diagnostic and prognostic marker of pancreatic adenocarcinoma.Cancer Manag Res. 2018 Jun 13;10:1507-1515. doi: 10.2147/CMAR.S158712. eCollection 2018. Cancer Manag Res. 2018. PMID: 29942152 Free PMC article.
-
ER Negative Breast Cancer and miRNA: There Is More to Decipher Than What the Pathologist Can See!Biomedicines. 2023 Aug 18;11(8):2300. doi: 10.3390/biomedicines11082300. Biomedicines. 2023. PMID: 37626796 Free PMC article. Review.
References
-
- Allan GJ, Beattie J, Flint DJ. 2004. The role of IGFBP-5 in mammary gland development and involution. Domest Anim Endocrinol 27: 257–266. - PubMed
-
- Aqeilan RI, Calin GA, Croce CM. 2010. miR-15a and miR-16-1 in cancer: discovery, function and future perspectives. Cell Death Differ 17: 215–220. - PubMed
-
- Baxter FO, Came PJ, Abell K, Kedjouar B, Huth M, Rajewsky K, Pasparakis M, Watson CJ. 2006. IKKβ/2 induces TWEAK and apoptosis in mammary epithelial cells. Development 133: 3485–3494. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
