A derivative of platelet-derived growth factor receptor alpha binds to the trimer of human cytomegalovirus and inhibits entry into fibroblasts and endothelial cells
- PMID: 28403220
- PMCID: PMC5389858
- DOI: 10.1371/journal.ppat.1006273
A derivative of platelet-derived growth factor receptor alpha binds to the trimer of human cytomegalovirus and inhibits entry into fibroblasts and endothelial cells
Abstract
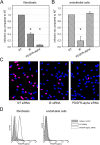
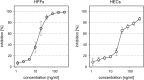
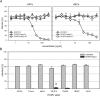
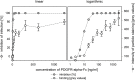
Human cytomegalovirus (HCMV) is a widely distributed herpesvirus that causes significant morbidity in immunocompromised hosts. Inhibitors of viral DNA replication are available, but adverse effects limit their use. Alternative antiviral strategies may include inhibition of entry. We show that soluble derivatives of the platelet-derived growth factor receptor alpha (PDGFR-alpha), a putative receptor of HCMV, can inhibit HCMV infection of various cell types. A PDGFR-alpha-Fc fusion protein binds to and neutralizes cell-free virus particles at an EC50 of 10-30 ng/ml. Treatment of particles reduced both attachment to and fusion with cells. In line with the latter, PDGFR-alpha-Fc was also effective when applied postattachment. A peptide scan of the extracellular domain of PDGFR-alpha identified a 40mer peptide that inhibits infection at an EC50 of 1-2 nmol/ml. Both, peptide and fusion protein, were effective against various HCMV strains and are hence promising candidates for the development of novel anti-HCMV therapies.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

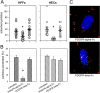
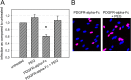
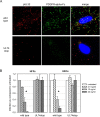

Similar articles
-
Human cytomegalovirus glycoprotein complex gH/gL/gO uses PDGFR-α as a key for entry.PLoS Pathog. 2017 Apr 12;13(4):e1006281. doi: 10.1371/journal.ppat.1006281. eCollection 2017 Apr. PLoS Pathog. 2017. PMID: 28403202 Free PMC article.
-
Platelet-derived growth factor-alpha receptor activation is required for human cytomegalovirus infection.Nature. 2008 Sep 18;455(7211):391-5. doi: 10.1038/nature07209. Epub 2008 Aug 13. Nature. 2008. PMID: 18701889
-
Transmission of cell-associated human cytomegalovirus isolates between various cell types using polymorphonuclear leukocytes as a vehicle.Med Microbiol Immunol. 2021 Aug;210(4):197-209. doi: 10.1007/s00430-021-00713-6. Epub 2021 Jun 6. Med Microbiol Immunol. 2021. PMID: 34091753 Free PMC article.
-
[Interrelationship between human cytomegalovirus infection and chemokine].Nihon Rinsho. 1998 Jan;56(1):69-74. Nihon Rinsho. 1998. PMID: 9465667 Review. Japanese.
-
Novel inhibitors of human CMV.Curr Opin Investig Drugs. 2008 Feb;9(2):132-45. Curr Opin Investig Drugs. 2008. PMID: 18246516 Review.
Cited by
-
Identification of adipocyte plasma membrane-associated protein as a novel modulator of human cytomegalovirus infection.PLoS Pathog. 2019 Jul 29;15(7):e1007914. doi: 10.1371/journal.ppat.1007914. eCollection 2019 Jul. PLoS Pathog. 2019. PMID: 31356650 Free PMC article.
-
Immunization of Rabbits with Recombinant Human Cytomegalovirus Trimeric versus Monomeric gH/gL Protein Elicits Markedly Higher Titers of Antibody and Neutralization Activity.Int J Mol Sci. 2019 Jun 28;20(13):3158. doi: 10.3390/ijms20133158. Int J Mol Sci. 2019. PMID: 31261659 Free PMC article.
-
Polymorphisms in Human Cytomegalovirus Glycoprotein O (gO) Exert Epistatic Influences on Cell-Free and Cell-to-Cell Spread and Antibody Neutralization on gH Epitopes.J Virol. 2020 Mar 31;94(8):e02051-19. doi: 10.1128/JVI.02051-19. Print 2020 Mar 31. J Virol. 2020. PMID: 31996433 Free PMC article.
-
Human Cytomegalovirus Tropism Modulator UL148 Interacts with SEL1L, a Cellular Factor That Governs Endoplasmic Reticulum-Associated Degradation of the Viral Envelope Glycoprotein gO.J Virol. 2018 Aug 29;92(18):e00688-18. doi: 10.1128/JVI.00688-18. Print 2018 Sep 15. J Virol. 2018. PMID: 29997207 Free PMC article.
-
Human cytomegalovirus glycoprotein variants governing viral tropism and syncytium formation in epithelial cells and macrophages.J Virol. 2024 Jul 23;98(7):e0029324. doi: 10.1128/jvi.00293-24. Epub 2024 Jun 5. J Virol. 2024. PMID: 38837351 Free PMC article.
References
-
- Gwee A, Curtis N, Connell TG, Garland S, Daley AJ. Ganciclovir for the treatment of congenital cytomegalovirus: what are the side effects? Pediatr Infect Dis J. 2014;33(1):115. Epub 2013/12/19. - PubMed
-
- Kropff B, Landini MP, Mach M. An ELISA using recombinant proteins for the detection of neutralizing antibodies against human cytomegalovirus. J Med Virol. 1993;39(3):187–95. Epub 1993/03/01. - PubMed
-
- Gerna G, Sarasini A, Patrone M, Percivalle E, Fiorina L, Campanini G, et al. Human cytomegalovirus serum neutralizing antibodies block virus infection of endothelial/epithelial cells, but not fibroblasts, early during primary infection. Journal of General Virology. 2008;89(4):853–65. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

