Human cytomegalovirus glycoprotein complex gH/gL/gO uses PDGFR-α as a key for entry
- PMID: 28403202
- PMCID: PMC5389851
- DOI: 10.1371/journal.ppat.1006281
Human cytomegalovirus glycoprotein complex gH/gL/gO uses PDGFR-α as a key for entry
Abstract
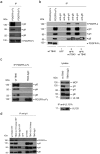
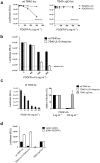
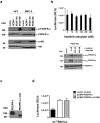
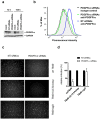
Herpesvirus gH/gL envelope glycoprotein complexes are key players in virus entry as ligands for host cell receptors and by promoting fusion of viral envelopes with cellular membranes. Human cytomegalovirus (HCMV) has two alternative gH/gL complexes, gH/gL/gO and gH/gL/UL128,130,131A which both shape the HCMV tropism. By studying binding of HCMV particles to fibroblasts, we could for the first time show that virion gH/gL/gO binds to platelet-derived growth factor-α (PDGFR-α) on the surface of fibroblasts and that gH/gL/gO either directly or indirectly recruits gB to this complex. PDGFR-α functions as an entry receptor for HCMV expressing gH/gL/gO, but not for HCMV mutants lacking the gH/gL/gO complex. PDGFR-α-dependent entry is not dependent on activation of PDGFR-α. We could also show that the gH/gL/gO-PDGFR-α interaction starts the predominant entry pathway for infection of fibroblasts with free virus. Cell-associated virus spread is either driven by gH/gL/gO interacting with PDGFR-α or by the gH/gL/UL128,130,131A complex. PDGFR-α-positive cells may thus be preferred first target cells for infections with free virus which might have implications for the design of future HCMV vaccines or anti-HCMV drugs.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Human Cytomegalovirus gH/gL/gO Promotes the Fusion Step of Entry into All Cell Types, whereas gH/gL/UL128-131 Broadens Virus Tropism through a Distinct Mechanism.J Virol. 2015 Sep;89(17):8999-9009. doi: 10.1128/JVI.01325-15. Epub 2015 Jun 17. J Virol. 2015. PMID: 26085146 Free PMC article.
-
The N Terminus of Human Cytomegalovirus Glycoprotein O Is Important for Binding to the Cellular Receptor PDGFRα.J Virol. 2019 May 15;93(11):e00138-19. doi: 10.1128/JVI.00138-19. Print 2019 Jun 1. J Virol. 2019. PMID: 30894468 Free PMC article.
-
Comparative analysis of gO isoforms reveals that strains of human cytomegalovirus differ in the ratio of gH/gL/gO and gH/gL/UL128-131 in the virion envelope.J Virol. 2013 Sep;87(17):9680-90. doi: 10.1128/JVI.01167-13. Epub 2013 Jun 26. J Virol. 2013. PMID: 23804643 Free PMC article.
-
Principles for studying in vivo attenuation of virus mutants: defining the role of the cytomegalovirus gH/gL/gO complex as a paradigm.Med Microbiol Immunol. 2015 Jun;204(3):295-305. doi: 10.1007/s00430-015-0405-2. Epub 2015 Mar 18. Med Microbiol Immunol. 2015. PMID: 25782576 Review.
-
Pathogen at the Gates: Human Cytomegalovirus Entry and Cell Tropism.Viruses. 2018 Dec 11;10(12):704. doi: 10.3390/v10120704. Viruses. 2018. PMID: 30544948 Free PMC article. Review.
Cited by
-
Human Cytomegalovirus Envelope Protein gpUL132 Regulates Infectious Virus Production through Formation of the Viral Assembly Compartment.mBio. 2020 Sep 29;11(5):e02044-20. doi: 10.1128/mBio.02044-20. mBio. 2020. PMID: 32994323 Free PMC article.
-
Growth Factor Receptor Signaling Inhibition Prevents SARS-CoV-2 Replication.Mol Cell. 2020 Oct 1;80(1):164-174.e4. doi: 10.1016/j.molcel.2020.08.006. Epub 2020 Aug 11. Mol Cell. 2020. PMID: 32877642 Free PMC article.
-
Deep Mutational Scanning of Viral Glycoproteins and Their Host Receptors.Front Mol Biosci. 2021 Apr 9;8:636660. doi: 10.3389/fmolb.2021.636660. eCollection 2021. Front Mol Biosci. 2021. PMID: 33898517 Free PMC article. Review.
-
A Native Human Monoclonal Antibody Targeting HCMV gB (AD-2 Site I).Int J Mol Sci. 2018 Dec 11;19(12):3982. doi: 10.3390/ijms19123982. Int J Mol Sci. 2018. PMID: 30544903 Free PMC article. Review.
-
Viral and Cellular Factors Contributing to the Hematogenous Dissemination of Human Cytomegalovirus via Polymorphonuclear Leukocytes.Viruses. 2022 Jul 18;14(7):1561. doi: 10.3390/v14071561. Viruses. 2022. PMID: 35891541 Free PMC article.
References
-
- Boppana SB, Britt WJ. Synopsis of clinical aspects of human cytomegalovirus disease In: Reddehase MJ, editors. Cytomegaloviruses: From Molecular Pathogenesis to Intervention. Norfolk, UK: Caister Academic Press; 2013; pp 1–25.
-
- Plachter B, Sinzger C, Jahn G. Cell types involved in replication and distribution of human cytomegalovirus. Adv Virus Res. 1996; 46:195–261. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

