Chk1 inhibition potentiates the therapeutic efficacy of PARP inhibitor BMN673 in gastric cancer
- PMID: 28401005
- PMCID: PMC5385637
Chk1 inhibition potentiates the therapeutic efficacy of PARP inhibitor BMN673 in gastric cancer
Abstract
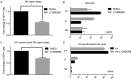
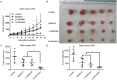
Globally, gastric cancer is the second leading cause of cancer deaths because of the lack of effective treatments for patients with advanced tumors when curative surgery is not possible. Thus, there is an urgent need to identify molecular targets in gastric cancer that can be used for developing novel therapies and prolonging patient survival. Checkpoint kinase 1 (Chk1) is a crucial regulator of cell cycle transition in DNA damage response (DDR). In our study, we report that Chk1 plays an important role in promoting gastric cancer cell survival and growth, which serves as an effective therapeutic target in gastric cancer. First, Chk1 ablation by small interfering RNA could significantly inhibit cell proliferation and sensitize the effects of ionizing radiation (IR) treatment in both p53 wild type gastric cancer cell line AGS, and p53 mutant cell line MKN1. Secondly, we tested the anticancer effects of Chk1 chemical inhibitor LY2606368, which is a novel Chk1/2 targeted drug undergoing clinical trials in many malignant diseases. We found that LY2606368 can induce DNA damage, and remarkably suppress cancer proliferation and induce apoptosis in AGS and MKN1 cells. Moreover, we identified that LY2606368 can significantly inhibit homologous recombination (HR) mediated DNA repair and thus showed marked synergistic anticancer effect in combination with poly (ADP-ribose) polymerase 1 (PARP1) inhibitor BMN673 in both in vitro studies and in vivo experiments using a gastric cancer PDx model. The synergy between LY2606368 and PARP1 was likely caused by impaired the G2M checkpoint due to LY2606368 treatment, which forced mitotic entry and cell death in the presence of BMN673. In conclusion, we propose that Chk1 is a valued target for gastric cancer treatment, especially Chk1 inhibitor combined with PARP inhibitor may be a more effective therapeutic strategy in gastric cancer.
Keywords: BMN673; Chk1; DNA damage response; LY2606368; gastric cancer.
Figures




Similar articles
-
PI3K p110α inhibition sensitizes cervical cancer cells with aberrant PI3K signaling activation to PARP inhibitor BMN673.Oncol Rep. 2019 Nov;42(5):2097-2107. doi: 10.3892/or.2019.7313. Epub 2019 Sep 13. Oncol Rep. 2019. PMID: 31545455
-
Augmented antitumor activity by olaparib plus AZD1775 in gastric cancer through disrupting DNA damage repair pathways and DNA damage checkpoint.J Exp Clin Cancer Res. 2018 Jun 28;37(1):129. doi: 10.1186/s13046-018-0790-7. J Exp Clin Cancer Res. 2018. PMID: 29954437 Free PMC article.
-
Prexasertib, a checkpoint kinase inhibitor: from preclinical data to clinical development.Cancer Chemother Pharmacol. 2020 Jan;85(1):9-20. doi: 10.1007/s00280-019-03950-y. Epub 2019 Sep 11. Cancer Chemother Pharmacol. 2020. PMID: 31512029 Review.
-
Prexasertib, a cell cycle checkpoint kinases 1 and 2 inhibitor, increases in vitro toxicity of PARP inhibition by preventing Rad51 foci formation in BRCA wild type high-grade serous ovarian cancer.Oncotarget. 2017 Oct 31;8(67):111026-111040. doi: 10.18632/oncotarget.22195. eCollection 2017 Dec 19. Oncotarget. 2017. PMID: 29340034 Free PMC article.
-
Moving From Poly (ADP-Ribose) Polymerase Inhibition to Targeting DNA Repair and DNA Damage Response in Cancer Therapy.J Clin Oncol. 2019 Sep 1;37(25):2257-2269. doi: 10.1200/JCO.18.02050. Epub 2019 May 3. J Clin Oncol. 2019. PMID: 31050911 Review.
Cited by
-
Loss of phosphatase CTDNEP1 potentiates aggressive medulloblastoma by triggering MYC amplification and genomic instability.Nat Commun. 2023 Feb 10;14(1):762. doi: 10.1038/s41467-023-36400-8. Nat Commun. 2023. PMID: 36765089 Free PMC article.
-
Clinical Implementation of Precision Medicine in Gastric Cancer.J Gastric Cancer. 2019 Sep;19(3):235-253. doi: 10.5230/jgc.2019.19.e25. Epub 2019 Aug 12. J Gastric Cancer. 2019. PMID: 31598369 Free PMC article. Review.
-
Multiomics analysis of serial PARP inhibitor treated metastatic TNBC inform on rational combination therapies.NPJ Precis Oncol. 2021 Oct 19;5(1):92. doi: 10.1038/s41698-021-00232-w. NPJ Precis Oncol. 2021. PMID: 34667258 Free PMC article.
-
Exploring the Synergy between PARP and CHK1 Inhibition in Matched BRCA2 Mutant and Corrected Cells.Cancers (Basel). 2020 Apr 4;12(4):878. doi: 10.3390/cancers12040878. Cancers (Basel). 2020. PMID: 32260355 Free PMC article.
-
Activity-based protein profiling and global proteome analysis reveal MASTL as a potential therapeutic target in gastric cancer.Cell Commun Signal. 2024 Aug 14;22(1):397. doi: 10.1186/s12964-024-01783-8. Cell Commun Signal. 2024. PMID: 39138495 Free PMC article.
References
-
- Tsai SH, Liu CA, Huang KH, Lan YT, Chen MH, Chao Y, Lo SS, Li AF, Wu CW, Chiou SH, Yang MH, Shyr YM, Fang WL. Advances in laparoscopic and robotic gastrectomy for gastric cancer. Pathol Oncol Res. 2017;23:13–17. - PubMed
-
- Venerito M, Linkw A, Rokkas T, Malfertheiner P. Gastric cancer-clinical and epidemiological aspects. Helicobacter. 2016;21(Suppl 1):39–44. - PubMed
-
- Ajani JA, D’Amico TA, Almhanna K, Bentrem DJ, Chao J, Das P, Denlinger CS, Fanta P, Farjah F, Fuchs CS, Gerdes H, Gibson M, Glasgow RE, Hayman JA, Hochwald S, Hofstetter WL, Ilson DH, Jaroszewski D, Johung KL, Keswani RN, Kleinberg LR, Korn WM, Leong S, Linn C, Lockhart AC, Ly QP, Mulcahy MF, Orringer MB, Perry KA, Poultsides GA, Scott WJ, Strong VE, Washington MK, Weksler B, Willett CG, Wright CD, Zelman D, McMillian N, Sundar H. Gastric cancer, version 3.2016, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2016;14:1286–1312. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
