A Peroxidase Peroxiredoxin 1-Specific Redox Regulation of the Novel FOXO3 microRNA Target let-7
- PMID: 28398822
- PMCID: PMC5695745
- DOI: 10.1089/ars.2016.6871
A Peroxidase Peroxiredoxin 1-Specific Redox Regulation of the Novel FOXO3 microRNA Target let-7
Abstract
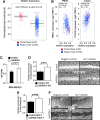
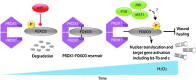
Precision in redox signaling is attained through posttranslational protein modifications such as oxidation of protein thiols. The peroxidase peroxiredoxin 1 (PRDX1) regulates signal transduction through changes in thiol oxidation of its cysteines. We demonstrate here that PRDX1 is a binding partner for the tumor suppressive transcription factor FOXO3 that directly regulates the FOXO3 stress response. Heightened oxidative stress evokes formation of disulfide-bound heterotrimers linking dimeric PRDX1 to monomeric FOXO3. Absence of PRDX1 enhances FOXO3 nuclear localization and transcription that are dependent on the presence of Cys31 or Cys150 within FOXO3. Notably, FOXO3-T32 phosphorylation is constitutively enhanced in these mutants, but nuclear translocation of mutant FOXO3 is restored with PI3K inhibition. Here we show that on H2O2 exposure, transcription of tumor suppressive miRNAs let-7b and let-7c is regulated by FOXO3 or PRDX1 expression levels and that let-7c is a novel target for FOXO3. Conjointly, inhibition of let-7 microRNAs increases let-7-phenotypes in PRDX1-deficient breast cancer cells. Altogether, these data ascertain the existence of an H2O2-sensitive PRDX1-FOXO3 signaling axis that fine tunes FOXO3 activity toward the transcription of gene targets in response to oxidative stress. Antioxid. Redox Signal. 28, 62-77.
Keywords: FOXO3; PRDX1; breast cancer; let-7; oxidative stress; tumor suppressor.
Conflict of interest statement
No competing financial interests exist.
Figures
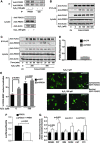
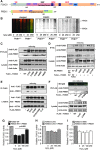
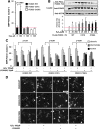

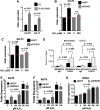
Similar articles
-
Peroxiredoxin 1 functions as a signal peroxidase to receive, transduce, and transmit peroxide signals in mammalian cells.Free Radic Biol Med. 2012 Oct 1;53(7):1522-30. doi: 10.1016/j.freeradbiomed.2012.08.001. Epub 2012 Aug 8. Free Radic Biol Med. 2012. PMID: 22902630
-
Redox proteomics reveal stress responsive proteins linking peroxiredoxin-1 status in glioma to chemosensitivity and oxidative stress.Biochim Biophys Acta. 2015 Jun;1854(6):624-31. doi: 10.1016/j.bbapap.2014.11.011. Epub 2014 Dec 4. Biochim Biophys Acta. 2015. PMID: 25484280
-
Cysteine Oxidation Targets Peroxiredoxins 1 and 2 for Exosomal Release through a Novel Mechanism of Redox-Dependent Secretion.Mol Med. 2015 Feb 13;21(1):98-108. doi: 10.2119/molmed.2015.00033. Mol Med. 2015. PMID: 25715249 Free PMC article.
-
Dual role of PRDX1 in redox-regulation and tumorigenesis: Past and future.Free Radic Biol Med. 2024 Jan;210:120-129. doi: 10.1016/j.freeradbiomed.2023.11.009. Epub 2023 Nov 15. Free Radic Biol Med. 2024. PMID: 37977211 Review.
-
Peroxiredoxin 1 - an antioxidant enzyme in cancer.J Cell Mol Med. 2017 Jan;21(1):193-202. doi: 10.1111/jcmm.12955. Epub 2016 Sep 21. J Cell Mol Med. 2017. PMID: 27653015 Free PMC article. Review.
Cited by
-
Peroxiredoxins wear many hats: Factors that fashion their peroxide sensing personalities.Redox Biol. 2021 Jun;42:101959. doi: 10.1016/j.redox.2021.101959. Epub 2021 Apr 20. Redox Biol. 2021. PMID: 33895094 Free PMC article. Review.
-
Electrophilic fatty acids impair RAD51 function and potentiate the effects of DNA-damaging agents on growth of triple-negative breast cells.J Biol Chem. 2019 Jan 11;294(2):397-404. doi: 10.1074/jbc.AC118.005899. Epub 2018 Nov 26. J Biol Chem. 2019. PMID: 30478172 Free PMC article.
-
Potential Cytoprotective and Regulatory Effects of Ergothioneine on Gene Expression of Proteins Involved in Erythroid Adaptation Mechanisms and Redox Pathways in K562 Cells.Genes (Basel). 2022 Dec 15;13(12):2368. doi: 10.3390/genes13122368. Genes (Basel). 2022. PMID: 36553634 Free PMC article.
-
Role of Cytosolic 2-Cys Prx1 and Prx2 in Redox Signaling.Antioxidants (Basel). 2019 Jun 10;8(6):169. doi: 10.3390/antiox8060169. Antioxidants (Basel). 2019. PMID: 31185618 Free PMC article. Review.
-
Temporal coordination of the transcription factor response to H2O2 stress.Nat Commun. 2024 Apr 23;15(1):3440. doi: 10.1038/s41467-024-47837-w. Nat Commun. 2024. PMID: 38653977 Free PMC article.
References
-
- Belguise K, Guo S, and Sonenshein GE. Activation of FOXO3a by the green tea polyphenol epigallocatechin-3-gallate induces estrogen receptor alpha expression reversing invasive phenotype of breast cancer cells. Cancer Res 67: 5763–5770, 2007 - PubMed
-
- Blake DC, Jr, Mikse OR, Freeman WM, and Herzog CR. FOXO3a elicits a pro-apoptotic transcription program and cellular response to human lung carcinogen nicotine-derived nitrosaminoketone (NNK). Lung Cancer 67: 37–47 - PubMed
-
- Boyerinas B, Park SM, Hau A, Murmann AE, and Peter ME. The role of let-7 in cell differentiation and cancer. Endocr Relat Cancer 17: F19–F36, 2010 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

