ERCC1-expressing circulating tumor cells as a potential diagnostic tool for monitoring response to platinum-based chemotherapy and for predicting post-therapeutic outcome of ovarian cancer
- PMID: 28388557
- PMCID: PMC5421848
- DOI: 10.18632/oncotarget.13286
ERCC1-expressing circulating tumor cells as a potential diagnostic tool for monitoring response to platinum-based chemotherapy and for predicting post-therapeutic outcome of ovarian cancer
Abstract
Background: We recently showed that the presence of ERCC1+CTCs is an independent predictive biomarker for platinum-resistance and poor prognosis of ovarian cancer. The goal of our current research was to determine how the auxiliary assessment of ERCC1-transcripts influences overall CTC-detection rate. We extended this investigation from an initially predictive setting to paired pre- and post-therapeutic blood analysis in order to see, whether ERCC1+CTCs dynamics mirror response to chemotherapy.
Methods: 65 Paired blood samples (10ml) of primary ovarian cancer patients at primary diagnosis and after chemotherapy were studied for CTCs with the AdnaTest Ovarian Cancer (QIAGEN Hannover GmbH). We analyzed the tumor-associated transcripts EpCAM, MUC-1 and CA-125. ERCC1-transcripts were investigated in a separate approach by singleplex RT-PCR.
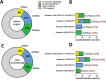
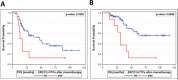
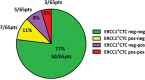
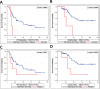
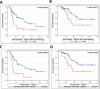
Results: Auxiliary assessment of ERCC1-transcripts enhanced the overall CTC-detection rate up to 17%. ERCC1+CTCs (defined as positive for one of the AdnaTest markers plus ERCC1-positivity) were detected in 15% of patients at primary diagnosis and in 12% after chemotherapy. The presence of ERCC1+CTCs after chemotherapy correlated with platinum-resistance (P=0.01), reduced PFS (P=0.0293) and OS (P=0.0008) and their persistence indicated poor post-therapeutic outcome (PFS: P=0.005; OS: P=0.0058). Interestingly, the assessment of ERCC1-transcripts alone was sufficient for the detection of prognostic relevant ERCC1-expressing CTCs.
Conclusion: Auxiliary assessment of ERCC1-transcripts expands the phenotypic spectrum of CTC detection and defines an additional overlapping fraction of ERCC1-expressing CTCs, which are potentially selected by platinum-based chemotherapy. Specifically, we suggest that ERCC1+CTCs could additionally be useful as a surrogate for monitoring platinum-based chemotherapy and to assess the post-therapeutic outcome of ovarian cancer.
Keywords: ERCC1; circulating tumor cells; ovarian cancer; platinum-resistance; prognosis.
Conflict of interest statement
Sabine Kasimir-Bauer is a consultant for QIAGEN Hannover GmbH. Siegfried Hauch is an employee of QIAGEN Hannover GmbH. All other authors have no conflicts of interest.
Figures
Similar articles
-
ERCC1-positive circulating tumor cells in the blood of ovarian cancer patients as a predictive biomarker for platinum resistance.Clin Chem. 2014 Oct;60(10):1282-9. doi: 10.1373/clinchem.2014.224808. Epub 2014 Jul 11. Clin Chem. 2014. PMID: 25015375
-
EMT-like circulating tumor cells in ovarian cancer patients are enriched by platinum-based chemotherapy.Oncotarget. 2017 Jul 25;8(30):48820-48831. doi: 10.18632/oncotarget.16179. Oncotarget. 2017. PMID: 28415744 Free PMC article.
-
Molecular profiling and prognostic relevance of circulating tumor cells in the blood of ovarian cancer patients at primary diagnosis and after platinum-based chemotherapy.Int J Gynecol Cancer. 2011 Jul;21(5):822-30. doi: 10.1097/IGC.0b013e318216cb91. Int J Gynecol Cancer. 2011. PMID: 21613958
-
[ERCC1 as a Marker of Ovarian Cancer Resistance to Platinum Drugs].Antibiot Khimioter. 2015;60(3-4):42-50. Antibiot Khimioter. 2015. PMID: 26415382 Review. Russian.
-
Association between the ERCC1 polymorphism and platinum-based chemotherapy effectiveness in ovarian cancer: a meta-analysis.BMC Womens Health. 2017 Jun 17;17(1):43. doi: 10.1186/s12905-017-0393-z. BMC Womens Health. 2017. PMID: 28623887 Free PMC article. Review.
Cited by
-
Circulating tumor cells and cell-free nucleic acids in patients with gynecological malignancies.Virchows Arch. 2018 Oct;473(4):395-403. doi: 10.1007/s00428-018-2447-5. Epub 2018 Aug 25. Virchows Arch. 2018. PMID: 30145616 Review.
-
Research Progress in Prognostic Factors and Biomarkers of Ovarian Cancer.J Cancer. 2021 May 13;12(13):3976-3996. doi: 10.7150/jca.47695. eCollection 2021. J Cancer. 2021. PMID: 34093804 Free PMC article. Review.
-
Liquid biopsy in ovarian cancer in China and the world: current status and future perspectives.Front Oncol. 2023 Dec 19;13:1276085. doi: 10.3389/fonc.2023.1276085. eCollection 2023. Front Oncol. 2023. PMID: 38169730 Free PMC article. Review.
-
Prediction of Chemoresistance-How Preclinical Data Could Help to Modify Therapeutic Strategy in High-Grade Serous Ovarian Cancer.Curr Oncol. 2023 Dec 29;31(1):229-249. doi: 10.3390/curroncol31010015. Curr Oncol. 2023. PMID: 38248100 Free PMC article. Review.
-
Osteopontin enhances cisplatin resistance of human A549 lung cancer cells via stimulating the PI3K signaling pathway and upregulating ERCC1 expression.Transl Cancer Res. 2020 May;9(5):3258-3265. doi: 10.21037/tcr.2020.03.60. Transl Cancer Res. 2020. PMID: 35117692 Free PMC article.
References
-
- Goodman MT, Howe HL, Tung KH, Hotes J, Miller BA, Coughlin SS, Chen VW. Incidence of ovarian cancer by race and ethnicity in the United States, 1992-1997. Cancer. 2003;97:2676–2685. - PubMed
-
- du Bois A, Quinn M, Thigpen T, Vermorken J, Avall- Lundqvist E, Bookman M, Bowtell D, Brady M, Casado A, Cervantes A, Eisenhauer E, Friedlaender M, Fujiwara K, et al. 2004 consensus statements on the management of ovarian cancer: final document of the 3rd International Gynecologic Cancer Intergroup Ovarian Cancer Consensus Conference (GCIG OCCC 2004) Ann Oncol. 2005;16:viii7–viii12. - PubMed
-
- du Bois A, Reuss A, Pujade-Lauraine E, Harter P, Ray-Coquard I, Pfisterer J. Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: a combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials: by the Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzinom (AGO-OVAR) and the Groupe d’Investigateurs Nationaux Pour les Etudes des Cancers de l’Ovaire (GINECO) Cancer. 2009;115:1234–1244. - PubMed
-
- Wimberger P, Lehmann N, Kimmig R, Burges A, Meier W, Du Bois A. Arbeitsgemeinschaft Gynaekologische Onkologie Ovarian Cancer Study G. Prognostic factors for complete debulking in advanced ovarian cancer and its impact on survival. An exploratory analysis of a prospectively randomized phase III study of the Arbeitsgemeinschaft Gynaekologische Onkologie Ovarian Cancer Study Group (AGO-OVAR) Gynecol Oncol. 2007;106:69–74. - PubMed
-
- Wimberger P, Wehling M, Lehmann N, Kimmig R, Schmalfeldt B, Burges A, Harter P, Pfisterer J, du Bois A. Influence of residual tumor on outcome in ovarian cancer patients with FIGO stage IV disease: an exploratory analysis of the AGO-OVAR (Arbeitsgemeinschaft Gynaekologische Onkologie Ovarian Cancer Study Group) Ann Surg Oncol. 2010;17:1642–1648. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

